One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ion-Modified Cellulose Acetate Light Conversion Film
2.2. Structural Characterization and Performance Testing
3. Results
3.1. Structure and Morphology Characterization of Films
3.2. Fluorescent Characterization of the Light Conversion Films
3.3. Performance Characterization of the Films
4. Conclusions
- (1)
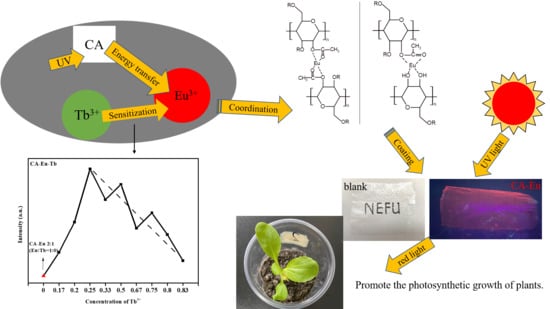
- Eu3+ reacted with CA and formed an Eu-O coordination bond with the O atoms in C=O, C–O (O=C–O), and O–H of CA structure. The organic ligand CA has a good absorption coefficient at UV wavelength range so that it could effectively transfer energy to Eu3+ through ultraviolet absorption and make CA–Eu light conversion film emit high-purity red light through 5D0→7F2 electric dipole transition. Due to the concentration quenching effect, the optimal fluorescence intensity was obtained at a CA: Eu3+ ratio of 3:1. In addition, the influence of the reaction time was also explored and the optimal fluorescence performance was obtained for the reaction time at 65 min.
- (2)
- After doping Tb3+ in the light-conversion films, the Tb3+ could effectively increase the fluorescence intensity of Eu3+ to play a sensitization role in the process of energy transfer.
- (3)
- The prepared light conversion film had a simple preparation process, high transparency, high tensile strength, and good flexibility. The material could absorb ultraviolet light and convert it into red light beneficial for the growth of plant photosynthesis. The material was also biodegradable, which is useful for efficiency and environmental protection.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plantcell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Liu, W.; Jia, X.; Ma, L.; Ren, L.; Xue, M.; Liu, X. Exploration of highly efficient light conversion agents for agricultural film based on the bay-substituted perylene diimides derivatives. Dye. Pigment. 2018, 159, 483–490. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Y.; Yu, Y.; Liu, Z.; Zhang, Y.; Qi, Y.; Zhou, C. Exploring highly efficient light conversion agents for agricultural film based on aggregation induced emission effects. J. Mater. Chem. C 2016, 4, 11291–11297. [Google Scholar] [CrossRef]
- Gong, T.; Li, Y.; Lei, B.; Zhang, X.; Liu, Y.; Zhang, H. Solid-state silicon nanoparticles with color-tunable photoluminescence and multifunctional applications. J. Mater. Chem. C 2019, 7, 5962–5969. [Google Scholar] [CrossRef]
- Hou, L.; Xi, J.; Chen, X.; Li, X.; Ma, W.; Lu, J.; Xu, J.; Lin, Y.B. Biodegradability and ecological impacts of polyethylene-based mulching film at agricultural environment. J. Hazard. Mater. 2019, 378, 120774. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, Z.-X.; Feng, L.-S.; Zheng, M.-Z.; Chi, D.-C.; Meng, W.-Z.; Hou, Z.-Y.; Bai, W.; Li, K.-Y. Plastic Film Mulching for Water-Efficient Agricultural Applications and Degradable Films Materials Development Research. Mater. Manuf. Process. 2015, 30, 143–154. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Jia, S.; Pan, H.; Zhang, H.; Wang, D.; Dong, L. Exploring polylactide/poly(butylene adipate-co-terephthalate)/rare earth complexes biodegradable light conversion agricultural films. Int. J. Biol. Macromol. 2019, 127, 210–221. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Ai, X.; Pan, H.; Zhang, H.; Dong, L. Polylactide/poly(butylene adipate-co-terephthalate)/rare earth complexes as biodegradable light conversion agricultural films. Polym. Adv. Technol. 2019, 30, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Zeshan, H.U.; Lanhua, L.I.; Chengbin, S.; Qiang, C.A.I. Light Conversion Film and Its Investigation Status. Mater. Rev. 2008, 22, 290–293. [Google Scholar]
- Zhao, X.; Huang, C.; Zhang, S.; Wang, C. Cellulose Acetate/Activated Carbon Composite Membrane with Effective Dye Adsorption Performance. J. Macromol. Sci. Part B-Phys. 2019, 58, 909–920. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Fan, Y.; Shi, H.; Wang, Y.; Ma, J.; Li, H. Novel dual superlyophobic cellulose membrane for multiple oil/water separation. Chemosphere 2020, 241, 125067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.-X.; Zhang, L.; Chen, Y.; Zhang, Q.-T. Co-luminescence properties of terbium ions-benzoic acid-phen complexes doped with europium ions. Rare Met. 2013, 32, 599–604. [Google Scholar] [CrossRef]
- Ye, J.; Wang, B.; Xiong, J. Effect of Eu(3+) Concentration on the Structure and Fluorescence Quenching of Carboxymethyl Cellulose /Eu(Ⅲ) Nanoparticles. Polym. Mater. Sci. Eng. 2016, 32, 32–37. [Google Scholar]
- Chen, M.; Li, R.-M.; Runge, T.; Feng, J.; Feng, J.; Hu, S.; Shi, Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 16971–16978. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation markers and plasticizer loss of cellulose acetate films during ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Jiang, L.; Li, K.; Yang, H.; Liu, X.; Li, W.; Xu, W.; Deng, B. Improving mechanical properties of electrospun cellulose acetate nanofiber membranes by cellulose nanocrystals with and without polyvinylpyrrolidone. Cellulose 2020, 27, 955–967. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Influence of pH and temperature of dip-coating solution on the properties of cellulose acetate-ceramic composite membrane for ultrafiltration. Carbohydr. Polym. 2018, 195, 613–621. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, Z.; Zhao, H.; Liang, R.; Wang, C.; Wang, D.; Li, J. Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III). Polymers 2020, 12, 893. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Yang, J.; Luo, D.; Su, X.; Qin, S. Construction of efficient desalting layer on a cellulose acetate membrane by acetalized surface crosslinking treatment. Polym. Eng. Sci. 2019, 59, 913–918. [Google Scholar] [CrossRef]
- Song, H.-m.; Zhu, L.-j.; Zeng, Z.-x.; Xue, Q.-j. High performance forward osmosis cellulose acetate (CA) membrane modified by polyvinyl alcohol and polydopamine. J. Polym. Res. 2018, 25, 159. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, B.; Xiong, J.; Sun, R. Enhanced fluorescence and structural characteristics of carboxymethyl cellulose/Eu(III) nano-complex: Influence of reaction time. Carbohydr. Polym. 2016, 135, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.R.; Zhang, Y.; Wu, A.; Yan, X.; Shen, S.; Ke, C.; Zhang, J. Preparation of microporous Cellulose/Poly(vinylidene fluoride-hexafluoropropylene) membrane for lithium ion batteries by phase inversion method. J. Power Sources 2018, 379, 197–205. [Google Scholar] [CrossRef]
- Cobo, F.N.; Faria-Tisher, P.C.S.; Duarte, J.L.; Carvalho, G.M. Preparation and characterization of microporous cellulose acetate films using breath figure method by spin coating technique. Cellulose 2017, 24, 4981–4995. [Google Scholar] [CrossRef]
- Mafirad, S.; Mehrnia, M.R.; Zahedi, P.; Hosseini, S.-N. Chitosan-based nanocomposite membranes with improved properties: Effect of cellulose acetate blending and TiO2 nanoparticles incorporation. Polym. Compos. 2018, 39, 4452–4466. [Google Scholar] [CrossRef]
- Sajjan, P.; Nayak, V.; Padaki, M.; Zadorozhnyy, V.Y.; Klyamkin, S.N.; Konik, P.A. Fabrication of Cellulose Acetate Film through Blending Technique with Palladium Acetate for Hydrogen Gas Separation. Energy Fuels 2020, 34, 11699–11707. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, B.; Sun, Y. Properties of Ultraviolet-Shielding Composite Film Prepared from Cellulose Acetate with Eu(III) Complex. Chemistryselect 2020, 5, 1688–1693. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Li, Q.; Zhang, X.; Li, T.; Li, P.; Yang, Z.; Guo, Q. Luminescent properties of Ba2SiO4:Eu3+ for white light emitting diodes. Phys. B Condens. Matter 2013, 411, 110–113. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Jung, H.C.; Park, J.Y.; Moon, B.K.; Balakrishnaiah, R.; Jeong, J.H.; Kim, J.H. The influence of sintering temperature on the photoluminescence properties of oxyapatite Eu3+:Ca2Gd8Si6O26 nanophosphors. Sens. Actuators B Chem. 2010, 146, 395–402. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, Y.N.; Li, Y.D. Preparation and characterization of ZrO2: Eu3+ phosphors. J. Alloys Compd. 2004, 381, 266–271. [Google Scholar] [CrossRef]
- Du, H.; Zhou, W.; Niu, J.; Xu, D.; Li, S.; Zhang, Z. Energy transfer and tunable photoluminescence of Sr6Gd2Na2(PO4)(6)F-2:Tb3+, Eu3+ phosphors for near-UV white LEDs. J. Mater. Sci. Mater. Electron. 2019, 30, 18575–18583. [Google Scholar] [CrossRef]
- Wu, J.; Hu, D. Synthesis of PAM membranes with different porous structure and their light-scattering behavior. In Proceedings of the 14th Colloid and Interface Chemistry Conference of the Chinese Chemical Society, Changchun, China, 21–25 July 2013; 2. [Google Scholar]
- Chen, H.; Li, R.; Xu, X.; Zhao, P.; Wong, D.S.H.; Chen, X.; Chen, S.; Yan, X. Citrate-based fluorophores in polymeric matrix by easy and green in situ synthesis for full-band UV shielding and emissive transparent display. J. Mater. Sci. 2019, 54, 1236–1247. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmol, G.; Gauss, C.; Fangueiro, R. Potential of Cellulose Microfibers for PHA and PLA Biopolymers Reinforcement. Molecules 2020, 25, 4653. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Iglesias, M.; Lizundia, E.; Lanceros-Mendez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. J Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Marlina, M.; Khalil, H.P.S.A.; Kurniawan, K.H.; Suyanto, H.; Hedwig, R.; Karnadi, I.; Olaiya, N.G.; Abdullah, C.K.; Abdulmadjid, S.N. Characterization and Performance Evaluation of Cellulose Acetate-Polyurethane Film for Lead II Ion Removal. Polymers 2020, 12, 1317. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Tang, R.; Yan, Q.; Tian, W.; Zhang, L. Enhanced permeability, mechanical and antibacterial properties of cellulose acetate ultrafiltration membranes incorporated with lignocellulose nanofibrils. Int. J. Biol. Macromol. 2020, 151, 159–167. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Xu, P.; Cen, C.; Chen, N.; Lin, H.; Wang, Q.; Xu, N.; Tang, J.; Teng, Z. Facile fabrication of silver nanoparticles deposited cellulose microfiber nanocomposites for catalytic application. J. Colloid Interface Sci. 2018, 526, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Z.; Dong, R.; Xie, G.; Zhou, J.; Wu, K.; Zhang, H.; Cai, Q.; Lei, B. Characterization and properties of a Sr2Si5N8:Eu2+-based light-conversion agricultural film. J. Rare Earths 2020, 38, 539–545. [Google Scholar] [CrossRef]











| Sample | T% Mean(200–350 nm) | T% Mean(350–700 nm) |
|---|---|---|
| CA | 54.3625 | 81.20739 |
| CA–Eu | 24.0450 | 83.23807 |
| CA–Eu–Tb | 30.5563 | 85.94716 |
| Sample (CA–Eu) | T% Mean(200–350 nm) | T% Mean(350–700 nm) |
|---|---|---|
| 10 μm | 43.78562 | 90.17846 |
| 20 μm | 37.51844 | 89.81298 |
| 24 μm | 29.26031 | 88.35093 |
| 72 μm | 26.37656 | 85.15506 |
| Sample | σM (MPa) | Mean/SD | εB (%) | Mean/SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| CA | 6.3744 | 13.0921 | 13.8606 | 12.2708 | 11.5697 | 11.4/3.0 | 2.8119 | 3.3088 | 1.8889 | 2.3977 | 3.2536 | 2.7/0.6 |
| CA-Eu | 16.2126 | 13.1485 | 9.6287 | 14.9523 | 12.7006 | 13.3/2.5 | 5.9627 | 5.309 | 4.9064 | 4.1741 | 3.8461 | 4.8/0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhao, Z.; Lu, Y.; Wang, D.; Wang, C.; Li, J. One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism. Polymers 2021, 13, 113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010113
Zhang Z, Zhao Z, Lu Y, Wang D, Wang C, Li J. One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism. Polymers. 2021; 13(1):113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010113
Chicago/Turabian StyleZhang, Zhihui, Zhengdong Zhao, Yujia Lu, Di Wang, Chengyu Wang, and Jian Li. 2021. "One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism" Polymers 13, no. 1: 113. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010113