Viscoelastic Relaxation of Polymerized Ionic Liquid and Lithium Salt Mixtures: Effect of Salt Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Materials
2.3. Blend Sample Preparation
2.4. Measurements
3. Results and Discussion
3.1. Miscibility of PC4-TFSI/LiTFSI Mixtures
3.2. Weight Fraction Dependence of Calorimetric Glass Transition Temperatures
3.3. Overview of the Chain Dynamics in PC4-TFST Molten System
3.4. LiTFSI Concentration Dependence of the Viscoelastic Spectra
3.5. WLF Analysis of the Rheological Shift Factors
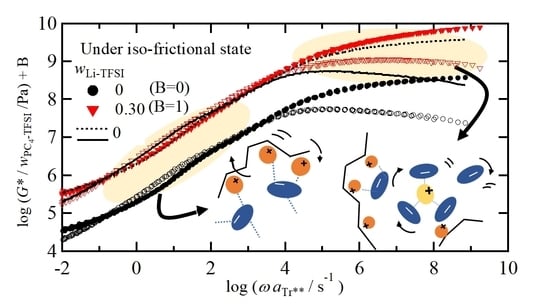
3.6. Chain Dynamics in Mixtures of PC4-TFSI/LiTFSI under Iso-Frictional Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| C1 (Equation (A2)) | C2/°C (Equation (A2)) | C2/°C with C1 = 7.62 | 104 αf/°C−1 | |
|---|---|---|---|---|
| 0 | 7.62 | 114 | 114 | 5.00 |
| 0.039 | 7.62 | 114 | 114 | 5.00 |
| 0.07 | 7.62 | 114 | 114 | 5.00 |
| 0.11 | 7.82 | 111 | 109 | 5.23 |
| 0.15 | 7.82 | 111 | 109 | 5.22 |
| 0.20 | 8.02 | 108 | 103 | 5.53 |
| 0.25 | 8.42 | 103 | 97.0 | 5.88 |
| 0.30 | 8.42 | 103 | 96.0 | 5.94 |
References
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Ohno, H. Molten salt type polymer electrolytes. Electrochim. Acta 2001, 46, 1407–1411. [Google Scholar] [CrossRef]
- Sangoro, J.R.; Iacob, C.; Agapov, A.L.; Wang, Y.; Berdzinski, S.; Rexhausen, H.; Strehmel, V.; Friedrich, C.; Sokolov, A.P.; Kremer, F. Decoupling of ionic conductivity from structural dynamics in polymerized ionic liquids. Soft Matter 2014, 10, 3536–3540. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.M.; Sun, J.K.; Yuan, J.Y. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.J.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly(ionic liquid)s: Synthesis, properties, and application. Polym. Sci. Ser. B 2016, 58, 73–142. [Google Scholar] [CrossRef]
- Rochow, E.T.; Coeler, M.; Pospiech, D.; Kobsch, O.; Mechtaeva, E.; Vogel, R.; Voit, B.; Nikolowski, K.; Wolter, M. In Situ Preparation of Crosslinked Polymer Electrolytes for Lithium Ion Batteries: A Comparison of Monomer Systems. Polymers 2020, 12, 1707. [Google Scholar] [CrossRef] [PubMed]
- Kalybekkyzy, S.; Kopzhassar, A.; Kahraman, M.V.; Mentbayeva, A.; Bakenov, Z. Fabrication of UV-Crosslinked Flexible Solid Polymer Electrolyte with PDMS for Li-Ion Batteries. Polymers 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa-Fujita, M.; Ishii, J.; Takeoka, Y.; Rikukawa, M. Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries. Polymers 2021, 13, 800. [Google Scholar] [CrossRef]
- Armand, M. Polymer Solid Electrolytes—An Overview. Solid State Ion. 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Robitaille, C.D.; Fauteux, D. Phase-Diagrams and Conductivity Characterization of Some Peo-Lix Electrolytes. J. Electrochem. Soc. 1986, 133, 315–325. [Google Scholar] [CrossRef]
- Watanabe, M.; Nagano, S.; Sanui, K.; Ogata, N. Ionic-Conductivity of Network Polymers from Poly(Ethylene Oxide) Containing Lithium Perchlorate. Polym. J. 1986, 18, 809–817. [Google Scholar] [CrossRef]
- Ratner, M.A.; Johansson, P.; Shriver, D.F. Polymer electrolytes: Ionic transport mechanisms and relaxation coupling. Mrs Bull. 2000, 25, 31–37. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D.; Henderson, W. Li+ cation environment, transport, and mechanical properties of the LiTFSI doped N-methyl-N-alkylpyrrolidinium +TFSI- ionic liquids. J. Phys. Chem. B 2006, 110, 16879–16886. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Balsara, N.P.; Kerr, J.B.; Inoue, T.; Watanabe, H. In situ dielectric characterization of poly(ethylene oxide) melts containing lithium perchlorate under steady shear flow. Macromolecules 2004, 37, 544–553. [Google Scholar] [CrossRef]
- Ohno, H.; Ito, K. Room-temperature molten salt polymers as a matrix for fast ion conduction. Chem. Lett. 1998, 751–752. [Google Scholar] [CrossRef]
- Wang, X.E.; Chen, F.F.; Girard, G.M.A.; Zhu, H.J.; MacFarlane, D.R.; Mecerreyes, D.; Armand, M.; Howlett, P.C.; Forsyth, M. Poly(Ionic Liquid)s-in-Salt Electrolytes with Co-coordination-Assisted Lithium-Ion Transport for Safe Batteries. Joule 2019, 3, 2687–2702. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Nasrabadi, A.T.; Aryal, D.; Ganesan, V. Mechanisms of Ion Transport in Lithium Salt-Doped Polymeric Ionic Liquid Electrolytes. Macromolecules 2020, 53, 6995–7008. [Google Scholar] [CrossRef]
- Castiglione, F.; Famulari, A.; Raos, G.; Meille, S.V.; Mele, A.; Appetecchi, G.B.; Passerini, S. Pyrrolidinium-Based Ionic Liquids Doped with Lithium Salts: How Does Li+ Coordination Affect Its Diffusivity? J. Phys. Chem. B 2014, 118, 13679–13688. [Google Scholar] [CrossRef]
- Nakamura, K.; Saiwaki, T.; Fukao, K.; Inoue, T. Viscoelastic Behavior of the Polymerized Ionic Liquid Poly(1-ethyl-3-vinylimidazolium bis(trifluoromethanesulfonylimide)). Macromolecules 2011, 44, 7719–7726. [Google Scholar] [CrossRef]
- Nakamura, K.; Fukao, K.; Inoue, T. Dielectric Relaxation and Viscoelastic Behavior of Polymerized Ionic Liquids with Various Counteranions. Macromolecules 2012, 45, 3850–3858. [Google Scholar] [CrossRef]
- Nakamura, K.; Fukao, K.; Inoue, T. Viscoelastic Behavior of Polymerized Ionic Liquids with Various Charge Densities. Nihon Reoroji Gakkaishi (J. Soc. Rheol. Jpn.) 2013, 41, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Inoue, T. Detailed Analysis of Sub-Rouse Mode Observed in Polymerized Ionic Liquids with Dynamic Birefringence Measurements. Nihon Reoroji Gakkaishi (J. Soc. Rheol. Jpn.) 2014, 42, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Matsumoto, A.; Nakamura, K. Dynamic Viscoelasticity and Birefringence of Poly(ionic liquids) in the Vicinity of Glass Transition Zone. Macromolecules 2013, 46, 6104–6109. [Google Scholar] [CrossRef]
- Fitzgerald, E.R.; Grandine, L.D.; Ferry, J.D. Dynamic Mechanical Properties of Polyisobutylene. J. Appl. Phys. 1953, 24, 650–655. [Google Scholar] [CrossRef]
- Okamoto, H.; Inoue, T.; Osaki, K. Viscoelasticity and Birefringence of Polyisobutylene. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1409–1416. [Google Scholar] [CrossRef]
- Ferry, J.D.; Grandine, L.D., Jr.; Fitzgerald, E.R. The Relaxation Distribution Function of Polyisobutylene in the Transition from Rubber-Like to Glass-Like Behavior. J. Appl. Phys. 1953, 24, 911–916. [Google Scholar] [CrossRef]
- Ngai, K.L.; Plazek, D.J. Resolution of Sub-Rouse Modes of Polystyrene by Dissolution. Macromolecules 2002, 35, 9136–9141. [Google Scholar] [CrossRef]
- Plazek, D.J.; Ngai, K.L. Correlation of Polymer Segmental Chain Dynamics with Temperature-Dependent Time-Scale Shifts. Macromolecules 1991, 24, 1222–1224. [Google Scholar] [CrossRef]
- Ferry, J.D.; Landel, R.F. Molecular friction coefficients in polymers and their temperature dependence. Kolloid Z. 1956, 148, 1–6. [Google Scholar] [CrossRef]
- Inoue, T.; Oba, N.; Urakawa, O. Reliability of Intrinsic Viscosity Estimated by Single Point Procedure at High Concentrations. Nihon Reoroji Gakkaishi (J. Soc. Rheol. Jpn.) 2014, 42, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Del Giudice, F.; Rotrattanadumrong, R.; Shen, A.Q. Rheological Scaling of Ionic-Liquid -Based Polyelectrolytes in Ionic Liquid Solutions. Macromolecules 2019, 52, 2759–2771. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Inoue, T.; Onogi, T.; Yao, M.L.; Osaki, K. Viscoelasticity of low molecular weight polystyrene. Separation of rubbery and glassy components. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 389–397. [Google Scholar] [CrossRef]
- Liu, C.Y.; He, J.S.; van Ruymbeke, E.; Keunings, R.; Bailly, C. Evaluation of different methods for the determination of the plateau modulus and the entanglement molecular weight. Polymer 2006, 47, 4461–4479. [Google Scholar] [CrossRef] [Green Version]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Colby, R.H.; Fetters, L.J.; Funk, W.G.; Graessley, W.W. Effects of Concentration and Thermodynamic Interaction on the Viscoelastic Properties of Polymer-Solutions. Macromolecules 1991, 24, 3873–3882. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. Mechanical Properties of Substances of High Molecular Weight 19. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Yan, Z.-C.; Zhang, B.-Q.; Liu, C.-Y. Dynamics of Concentrated Polymer Solutions Revisited: Isomonomeric Friction Adjustment and Its Consequences. Macromolecules 2014, 47, 4460–4470. [Google Scholar] [CrossRef]
- Urakawa, O.; Ikuta, H.; Maji, S.; Inoue, T. Rheological Behavior of Weakly Associated Polymers. Nihon Reoroji Gakkaishi (J. Soc. Rheol. Jpn.) 2018, 46, 131–137. [Google Scholar] [CrossRef]
- Angell, C.A. Formation of glasses from liquids and biopolymers. Science 1995, 267, 1924–1935. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Urakawa, O.; Adachi, K. Effect of local heterogeneity on dielectric relaxation spectra in concentrated solutions of poly(vinyl acetate) and poly(vinyl octanoate). Macromolecules 2000, 33, 7898–7904. [Google Scholar] [CrossRef]
- Yada, M.; Nakazawa, M.; Urakawa, O.; Morishima, Y.; Adachi, K. Effect of local heterogeneity on dielectric segmental relaxation of poly(vinyl acetate) in concentrated solution. Macromolecules 2000, 33, 3368–3374. [Google Scholar] [CrossRef]
- Nobukawa, S.; Urakawa, O.; Shikata, T.; Inoue, T. Cooperative Dynamics in Polystyrene and Low-Mass Molecule Mixtures. Macromolecules 2011, 44, 8324–8332. [Google Scholar] [CrossRef]
- Nobukawa, S.; Urakawa, O.; Shikata, T.; Inoue, T. Dynamics of a Probe Molecule Dissolved in Several Polymer Matrices with Different Side-Chain Structures: Determination of Correlation Length Relevant to Glass Transition. Macromolecules 2013, 46, 2206–2215. [Google Scholar] [CrossRef]
- Yasue, A.; Urakawa, O. Glass Transition Behaviors of Poly(Vinyl Pyridine)/Poly(Vinyl Phenol) Revisited. Polymers 2019, 11, 1153. [Google Scholar] [CrossRef] [Green Version]
- Urakawa, O.; Yasue, A.; Inoue, T. Rheological Behavior of Hydrogen Bonding Miscible Blend: High Molecular Weight Poly(2-vinyl pyridine) and Low Molecular Weight Poly(4-vinyl phenol). Nihon Reoroji Gakkaishi (J. Soc. Rheol. Jpn.) 2021, 49, 155–162. (in press). [Google Scholar] [CrossRef]











| Tr*/°C | Tr**/°C | |
|---|---|---|
| 0 | 100 | 100 |
| 0.039 | 93.9 | 93.9 |
| 0.07 | 89.2 | 89.2 |
| 0.11 | 83.8 | 80.9 |
| 0.15 | 84.1 | 81.1 |
| 0.20 | 83.7 | 78.0 |
| 0.25 | 79.5 | 68.7 |
| 0.30 | 75.1 | 64.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokokoji, A.; Kitayama, W.; Wichai, K.; Urakawa, O.; Matsumoto, A.; Vao-Soongnern, V.; Inoue, T. Viscoelastic Relaxation of Polymerized Ionic Liquid and Lithium Salt Mixtures: Effect of Salt Concentration. Polymers 2021, 13, 1772. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111772
Yokokoji A, Kitayama W, Wichai K, Urakawa O, Matsumoto A, Vao-Soongnern V, Inoue T. Viscoelastic Relaxation of Polymerized Ionic Liquid and Lithium Salt Mixtures: Effect of Salt Concentration. Polymers. 2021; 13(11):1772. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111772
Chicago/Turabian StyleYokokoji, Arisa, Wakana Kitayama, Kamonthira Wichai, Osamu Urakawa, Atsushi Matsumoto, Visit Vao-Soongnern, and Tadashi Inoue. 2021. "Viscoelastic Relaxation of Polymerized Ionic Liquid and Lithium Salt Mixtures: Effect of Salt Concentration" Polymers 13, no. 11: 1772. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111772