The Influences of 1-Butyl-3-Methylimidazolium Tetrafluoroborate on Electrochemical, Thermal and Structural Studies as Ionic Liquid Gel Polymer Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Ionic Liquid Gel Polymer Electrolyte
2.3. Characterizations
3. Results and Discussion
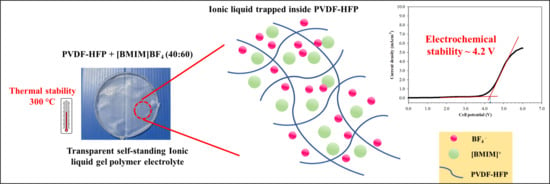
3.1. Physical Observation
3.2. Dielectric-Temperature-Dependent Studies
3.3. Electrochemical Stability Study—Linear Sweep Voltammetry
3.4. Thermal Analysis (DSC and TGA)
3.4.1. Differential Scanning Calorimetry
3.4.2. Thermogravimetric Analysis
3.5. Structural Analysis (X-Ray Diffractometry)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savinell, R.F.; Ota, K.I.; Kreysa, G. Encyclopedia of Applied Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Tran, Q.C.; Bui, V.T.; Dao, V.D.; Lee, J.K.; Choi, H.S. Ionic Liquid-based Polymer Electrolytes via Surfactant assisted Polymerization at the Plasma-Liquid Interface. Appl. Mater. Interfaces 2016, 8, 16125–16135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, N.; Qiu, Y.; Liu, R.; Hao, Z. Experimental Investigation on the High-frequency Pressure Oscillation Characteristics of a Combustion Process in a DI Diesel Engine. Energies 2020, 13, 871. [Google Scholar] [CrossRef] [Green Version]
- Dao, V.D.; Vu, N.H.; Yun, S. Recent advances and challenges for solar-driven water evaporation system toward applications. Nano Energy 2020, 68, 104324. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Fenton, D.E. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Lee, T.K.; Zaini, N.F.M.; Mobarak, N.N.; Hassan, N.H.; Noor, S.A.M.; Mamat, S.; Loh, K.S.; Bulat, K.H.K.; Su’ait, M.S.; Ahmad, A. PEO based polymer electrolyte comprised of epoxidized natural rubber material (ENR50) for Li-Ion polymer battery application. Electrochim. Acta 2019, 316, 283–291. [Google Scholar] [CrossRef]
- Kim, H.S.; Periasamy, P.; Moon, S.I. Electrochemical properties of the Li-ion polymer batteries with P (VdF-co-HFP)-based gel polymer electrolyte. J. Power Sources 2005, 141, 293–297. [Google Scholar] [CrossRef]
- Groce, F.; Gerace, F.; Dautzemberg, G.; Passerini, S.; Appetecchi, G.B.; Scrosati, B. Synthesis and characterization of highly conducting gel electrolytes. Electrochim. Acta 1994, 39, 2187–2194. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Idris, R.; Glasse, M.D.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Polymer electrolytes based on modified natural rubber for use in rechargeable lithium batteries. J. Power Sources 2001, 94, 206–211. [Google Scholar] [CrossRef]
- Benedict, T.J.; Banumathi, S.; Veluchamy, A.; Gangadharan, R.; Ahamad, A.Z.; Rajendran, S. Characterization of plasticized solid polymer electrolyte by XRD and AC impedance methods. J. Power Sources 1998, 75, 171–174. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Kim, K.S.; Shin, B.K.; Lee, H. Physical and electrochemical properties of 1-butyl-3-methylimidazolium bromide, 1-butyl-3-methylimidazolium iodide, and 1-butyl-3-methylimidazolium tetrafluoroborate. Korean J. Chem. Eng. 2004, 21, 1010–1014. [Google Scholar] [CrossRef]
- Yang, P.; Liu, L.; Li, L.; Hou, J.; Xu, Y.; Ren, X.; An, M.; Li, N. Gel polymer electrolyte based on polyvinylidenefluoride-co-hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim. Acta 2014, 115, 454–460. [Google Scholar] [CrossRef]
- Jayaraman, R.; Vickraman, P.; Subramanian, N.M.V.; Justin, A.S. AC impedance, XRD, DSC, FTIR studies on PbTiO3 dispersoid pristine PVdF-co-HFP and PEMA blended PVdF-co-HFP microcomposite electrolytes. J. Non Cryst. Solids 2016, 435, 27–32. [Google Scholar] [CrossRef]
- Saroj, A.L.; Krishnamoorthi, S.; Singh, R.K. Structural, thermal, and electrical transport behaviour of polymer electrolytes based on PVA and imidazolium based ionic liquid. J. Non Cryst. Solids 2017, 473, 87–95. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Electrical, mechanical, structural, and thermal behaviors of polymeric gel electrolyte membranes of poly (vinylidene fluoride-co-hexafluoropropylene) with the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate plus lithium tetrafluoroborate. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Z.; Li, Z.; He, Y.; Liu, Y.; Wang, H. PVDF-HFP composite polymer electrolyte with excellent electrochemical properties for Li-ion batteries. J. Solid State Electrochem. 2008, 12, 1497–1502. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.Y.; Choi, S.; Lee, H. Ionic liquid–polymer gel electrolytes based on morpholinium salt and PVdF (HFP) copolymer. J. Power Sources 2006, 155, 385–390. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Thermal stability, complexing behavior, and ionic transport of polymeric gel membranes based on polymer PVdF-HFP and ionic liquid, [BMIM][BF4]. J. Phys. Chem. B 2013, 117, 897–906. [Google Scholar]
- Orhan, O.Y.; Alper, E. Kinetics of reaction between CO2 and ionic liquid-carbon dioxide binding organic liquid hybrid systems: Analysis of gas-liquid absorption and stopped flow experiments. Chem. Eng. Sci. 2017, 170, 36–47. [Google Scholar] [CrossRef]
- Jagananthan, J.R.; Sivapragasm, M.; Wilfred, C.D. Thermal Characteristics of 1-Butyl-3-Methylimimidazolium Based Oxidant Ionic Liquids. J. Chem. Eng. Process Technol. 2016, 7, 1–6. [Google Scholar]
- Dzulkipli, M.Z.; Ahmad, A.; Su’ait, M.S.; Noor, S.A.M.; Dzulkurnain, N.A.; Karim, J.; Hassan, N.H. Studies on electrochemical behaviour of PVdF-HFP based ionic liquid gel polymer electrolyte. In Proceedings of the AIP Conference Proceedings, Universiti Kebangsaan Malaysia, Bangi, Malaysia, 4–5 April 2018; Volume 2111, p. 050006. [Google Scholar]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Dielectric properties and morphology of polymer electrolyte based on poly (ε-caprolactone) and ammonium thiocyanate. J. Non-Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Gohel, K.; Kanchan, D.K. Ionic conductivity, and relaxation studies in PVDF-HFP: PMMA-based gel polymer blend electrolyte with LiClO4 salt. J. Adv. Dielectr. 2018, 8, 1850005. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, J.R. Emphasizing solid materials and systems. In Impedance Spectroscopy; John Wiley & Sons Inc.: New York, NY, USA, 1987. [Google Scholar]
- Karmakar, A.; Ghosh, A. Dielectric permittivity and electric modulus of polyethylene oxide (PEO)–LiClO4 composite electrolytes. Curr. Appl. Phys. 2012, 12, 539–543. [Google Scholar] [CrossRef]
- Whba, R.; Su’ait, M.S.; TianKhoon, L.; Ibrahim, S.; Mohamed, N.S.; Ahmad, A. Free-Radical Photopolymerization of Acrylonitrile Grafted onto Epoxidized Natural Rubber. Polymers 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, A.; Pissis, P.; Grammatikakis, J. Dielectric relaxation spectroscopy in poly (hydroxyethyl acrylates)/water hydrogels. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1737–1750. [Google Scholar] [CrossRef]
- Gurusiddappa, J.; Madhuri, W.; Suvarna, R.P.; Dasan, K.P. Conductivity and dielectric behavior of polyethylene oxide-lithium perchlorate solid polymer electrolyte films. Indian J. Adv. Chem. Sci. 2016, 4, 14–19. [Google Scholar]
- Tripathi, S.K.; Gupta, A.; Kumari, M. Studies on electrical conductivity and dielectric behaviour of PVdF–HFP–PMMA–NaI polymer blend electrolyte. Bull. Mater. Sci. 2012, 35, 969–975. [Google Scholar] [CrossRef]
- Deraman, S.K.; Mohamed, N.S.; Subban, R.H.Y. Ionic liquid incorporated PVC based polymer electrolytes: Electrical and dielectric properties. Sains Malays. 2014, 43, 877–883. [Google Scholar]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater. Chem. Phys. 2009, 115, 557–561. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Effect of salt concentration on dielectric properties of Li-ion conducting blend polymer electrolytes. J. Mater. Sci. Mater. Electron. 2018, 29, 17903–17920. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, W.; Maass, P. Non-Debye relaxations in disordered ionic solids. Chem. Phys. 2002, 284, 439–467. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ion. 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Khoon, L.T.; Fui ML, W.; Hassan, N.H.; Su’ait, M.S.; Vedarajan, R.; Matsumi, N.; Kassim, M.B.; Shyuan, L.K.; Ahmad, A. In situ sol–gel preparation of ZrO2 in nano-composite polymer electrolyte of PVDF-HFP/MG49 for lithium-ion polymer battery. J. Sol. Gel. Sci. Technol. 2019, 90, 665–675. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, K.; Kim, H.S.; Kim, D.; Vikraman, D.; Prasanna, K.; Kathalingam, A.; Ramakant, S.; Rhee, H.W. An enhanced electrochemical and cycling properties of novel boronic Ionic liquid based ternary gel polymer electrolytes for rechargeable Li/LiCoO 2 cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kimura, H.; Okamoto, C.; Miyashita, T.; Imai, Y.; Abe, H. Glass transition behaviour of ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate–H2O mixed solutions. J. Chem. Thermodyn. 2011, 43, 410–412. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.M.; Nieman, R.A.; Angell, C.A. Ionic liquids of chelated orthoborates as model ionic glassformers. J. Phys. Chem. B 2003, 107, 11749–11756. [Google Scholar] [CrossRef]
- Shamim, N.; McKenna, G.B. Glass dynamics and anomalous aging in a family of ionic liquids above the glass transition temperature. J. Phys. Chem. B 2010, 114, 15742–15752. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Liew, C.; Ramesh, K. Evaluation and investigation on the effect of ionic liquid onto PMMA-PVC gel polymer blend electrolytes. J. Non-Cryst. Solids 2011, 357, 2132–2138. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- UK Essays. Polymer: The Glass Transition, November 2018. Available online: https://www.ukessays.com/essays/chemistry/polymer-the-glass-transition.php?vref=1 (accessed on 14 April 2021).
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J. Power Sources 2009, 187, 627–634. [Google Scholar] [CrossRef]
- Balo, L.; Gupta, H.; Singh, V.K.; Singh, R.K. Mixed anion effect on the ionic transport behavior, complexation and various physicochemical properties of ionic liquid based polymer gel electrolyte membranes. RSC Adv. 2016, 6, 73028–73039. [Google Scholar]
- Behrens, M.; Cross, J.S.; Akasaka, H.; Ohtake, N. Pyrolysis of cellulose mixed with ionic liquids 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide [bmim][TFSI], 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4], and 1-butyl-2, 3-dimethylimidazolium tetrafluoroborate [bmmim][BF4]. Biomass Bioenergy 2019, 122, 290–297. [Google Scholar] [CrossRef]
- Ataollahi, N.; Ahmad, A.; Lee, T.K.; Abdullah, A.R.; Rahman, M.Y.A. Preparation and characterization of PVDF-MG49-NH4 CF3SO3 based solid polymer electrolyte. e-Polymers 2014, 14, 115–120. [Google Scholar] [CrossRef]
- Gupta, H.; Balo, L.; Singh, V.K.; Chaurasia, S.K.; Singh, R.K. Effect of phosphonium based ionic liquid on structural, electrochemical and thermal behaviour of polymer poly (ethylene oxide) containing salt lithium bis (trifluoromethylsulfonyl) imide. RSC Adv. 2016, 6, 87878–87887. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzulkipli, M.Z.; Karim, J.; Ahmad, A.; Dzulkurnain, N.A.; Su’ait, M.S.; Yoshizawa-Fujita, M.; Tian Khoon, L.; Hassan, N.H. The Influences of 1-Butyl-3-Methylimidazolium Tetrafluoroborate on Electrochemical, Thermal and Structural Studies as Ionic Liquid Gel Polymer Electrolyte. Polymers 2021, 13, 1277. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081277
Dzulkipli MZ, Karim J, Ahmad A, Dzulkurnain NA, Su’ait MS, Yoshizawa-Fujita M, Tian Khoon L, Hassan NH. The Influences of 1-Butyl-3-Methylimidazolium Tetrafluoroborate on Electrochemical, Thermal and Structural Studies as Ionic Liquid Gel Polymer Electrolyte. Polymers. 2021; 13(8):1277. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081277
Chicago/Turabian StyleDzulkipli, Mariah Zuliana, Jamilah Karim, Azizan Ahmad, Nurul Akmaliah Dzulkurnain, Mohd Sukor Su’ait, Masahiro Yoshizawa-Fujita, Lee Tian Khoon, and Nur Hasyareeda Hassan. 2021. "The Influences of 1-Butyl-3-Methylimidazolium Tetrafluoroborate on Electrochemical, Thermal and Structural Studies as Ionic Liquid Gel Polymer Electrolyte" Polymers 13, no. 8: 1277. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081277