Atomic Scale Mechanisms Controlling the Oxidation of Polyethylene: A First Principles Study
Abstract
:1. Introduction
- (i)
- The capture of oxygen by an alkyl radical;
- (ii)
- The formation of hydroperoxides;
- (iii)
- The decomposition of the latter.
2. Materials and Methods
3. Results
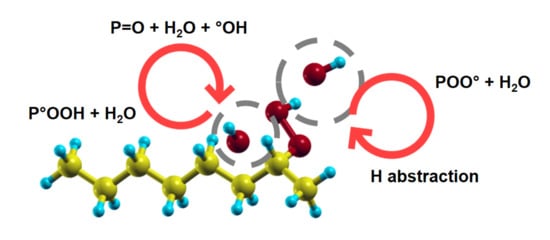
3.1. Oxygen Capture by an Alkyl Radical
3.2. Formation of Hydroperoxides
3.3. Decomposition of Hydroperoxides
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, 27, R292–R293. [Google Scholar] [CrossRef] [Green Version]
- Peacock, A.J. Handbook of Polyethylene; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2000. [Google Scholar]
- Fang, L.; Leng, Y.; Gao, P. Processing of hydroxyapatite reinforced ultrahigh molecular weight polyethylene for biomedical applications. Biomaterials 2005, 26, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celina, M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M. Fundamentals of Polymer Degradation and Stabilisation; Elsevier Applied Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Martinez-Vega, J. (Ed.) Dielectric Materials for Electrical Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. II. The kinetics of oxidation of unconjugated olefins. Trans. Faraday Soc. 1946, 42, 236–243. [Google Scholar] [CrossRef]
- Tobolsky, A.V.; Metz, D.J.; Mesrobian, R.B. Low Temperature Autoxidation of Hydrocarbons: The Phenomenon of Maximum Rates1,2. J. Am. Chem. Soc. 1950, 72, 1942–1952. [Google Scholar] [CrossRef]
- Decker, C.; Mayo, F.R.; Richardson, H. Aging and degradation of polyolefins. III. Polyethylene and ethylene–propylene copolymers. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 2879–2898. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1973.170111110 (accessed on 21 June 2021). [CrossRef]
- Somersall, A.C.; Guillet, J.E. Computer Modeling Studies of Polymer Photooxidation and Stabilization. In Proceedings of the Polymer Stabilization and Degradation, St Louis, MO, USA, 9–12 April 1984; pp. 211–234. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/pdf/10.1021/bk-1985-0280.ch016 (accessed on 21 June 2021).
- Gillen, K.T.; Clough, R.L. A kinetic model for predicting oxidative degradation rates in combined radiation-thermal environments. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 2683–2707. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1985.170231011 (accessed on 21 June 2021). [CrossRef]
- Gillen, K.T.; Wise, J.; Clough, R.L. General solution for the basic autoxidation scheme. Polym. Degrad. Stab. 1995, 47, 149–161. [Google Scholar] [CrossRef]
- Seguchi, T.; Tamura, K.; Ohshima, T.; Shimada, A.; Kudoh, H. Degradation mechanisms of cable insulation materials during radiation–thermal ageing in radiation environment. Radiat. Phys. Chem. 2011, 80, 268–273. [Google Scholar] [CrossRef]
- Khelidj, N.; Colin, X.; Audouin, L.; Verdu, J.; Monchy-Leroy, C.; Prunier, V. Oxidation of polyethylene under irradiation at low temperature and low dose rate. Part I. The case of “pure” radiochemical initiation. Polym. Degrad. Stab. 2006, 91, 1593–1597. [Google Scholar] [CrossRef]
- Niki, E.; Decker, C.; Mayo, F.R. Aging and degradation of polyolefins. I. Peroxide-initiated oxidations of atactic polypropylene. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 2813–2845. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1973.170111108 (accessed on 21 June 2021). [CrossRef]
- Costa, L.; Luda, M.P.; Trossarelli, L. Ultra high molecular weight polyethylene—II. Thermal- and photo-oxidation. Polym. Degrad. Stab. 1997, 58, 41–54. [Google Scholar] [CrossRef]
- Salvalaggio, M.; Bagatin, R.; Fornaroli, M.; Fanutti, S.; Palmery, S.; Battistel, E. Multi-component analysis of low-density polyethylene oxidative degradation. Polym. Degrad. Stab. 2006, 91, 2775–2785. [Google Scholar] [CrossRef]
- Fodor, Z.; Iring, M.; Tüdős, F.; Kelen, T. Determination of carbonyl-containing functional groups in oxidized polyethylene. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 2539–2550. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1984.170221021 (accessed on 21 June 2021). [CrossRef]
- Da Cruz, M.; Van Schoors, L.; Benzarti, K.; Colin, X. Thermo-oxidative degradation of additive free polyethylene. Part I. Analysis of chemical modifications at molecular and macromolecular scales. J. Appl. Polym. Sci. 2016, 133. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/app.43287 (accessed on 21 June 2021). [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Khelidj, N.; Colin, X.; Audouin, L.; Verdu, J.; Monchy-Leroy, C.; Prunier, V. Oxidation of polyethylene under irradiation at low temperature and low dose rate. Part II. Low temperature thermal oxidation. Polym. Degrad. Stab. 2006, 91, 1598–1605. [Google Scholar] [CrossRef]
- Colin, X.; Monchy-Leroy, C.; Audouin, L.; Verdu, J. Lifetime prediction of polyethylene in nuclear plants. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 251–255. [Google Scholar] [CrossRef]
- Colin, X.; Richaud, E.; Verdu, J.; Monchy-Leroy, C. Kinetic modelling of radiochemical ageing of ethylene–propylene copolymers. Radiat. Phys. Chem. 2010, 79, 365–370. [Google Scholar] [CrossRef]
- Colin, X.; Fayolle, B.; Audouin, L.; Verdu, J. About a quasi-universal character of unstabilised polyethylene thermal oxidation kinetics. Polym. Degrad. Stab. 2003, 80, 67–74. [Google Scholar] [CrossRef]
- Colin, X.; Audouin, L.; Verdu, J. Determination of thermal oxidation rate constants by an inverse method. Application to polyethylene. Polym. Degrad. Stab. 2004, 86, 309–321. [Google Scholar] [CrossRef]
- Colin, X.; Fayolle, B.; Audouin, L.; Verdu, J. The classical kinetic model for radical chain oxidation of hydrocarbon substrates initiated by bimolecular hydroperoxide decomposition. Int. J. Chem. Kinet. 2006, 38, 666–676. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/kin.20201 (accessed on 21 June 2021). [CrossRef]
- Hayes, C.J.; Burgess, D.R. Kinetic Barriers of H-Atom Transfer Reactions in Alkyl, Allylic, and Oxoallylic Radicals as Calculated by Composite Ab Initio Methods. J. Phys. Chem. A 2009, 113, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.T.; Hamilton, I.P.; Pritchard, H.O. Self-abstraction in aliphatic hydroperoxyl radicals. J. Chem. Soc. Faraday Trans. 1998, 94, 2303–2306. [Google Scholar] [CrossRef]
- Kysel, O.; Budzák, Š.; Medved, M.; Mach, P. A DFT study of H-isomerisation in alkoxy-, alkylperoxy- and alkyl radicals: Some implications for radical chain reactions in polymer systems. Polym. Degrad. Stab. 2011, 96, 660–669. [Google Scholar] [CrossRef]
- Oluwoye, I.; Altarawneh, M.; Gore, J.; Dlugogorski, B.Z. Oxidation of crystalline polyethylene. Combust. Flame 2015, 162, 3681–3690. [Google Scholar] [CrossRef]
- Pfaendtner, J.; Yu, X.; Broadbelt, L.J. Quantum Chemical Investigation of Low-Temperature Intramolecular Hydrogen Transfer Reactions of Hydrocarbons. J. Phys. Chem. A 2006, 110, 10863–10871. [Google Scholar] [CrossRef]
- De Sainte Claire, P. Degradation of PEO in the Solid State: A Theoretical Kinetic Model. Macromolecules 2009, 42, 3469–3482. [Google Scholar] [CrossRef] [Green Version]
- Ceresoli, D.; Tosatti, E.; Scandolo, S.; Santoro, G.; Serra, S. Trapping of excitons at chemical defects in polyethylene. J. Chem. Phys. 2004, 121, 6478–6484. [Google Scholar] [CrossRef] [Green Version]
- Unge, M.; Törnkvist, C.; Christen, T. Space charges and deep traps in polyethylene—Ab initio simulations of chemical impurities and defects. In Proceedings of the 2013 IEEE International Conference on Solid Dielectrics (ICSD), Bologna, Italy, 30 June–4 July 2013; pp. 935–939. [Google Scholar] [CrossRef]
- Huzayyin, A.; Boggs, S.; Ramprasad, R. Quantum mechanical studies of carbonyl impurities in dielectric polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 920–925. [Google Scholar] [CrossRef]
- Roma, G.; Bruneval, F.; Martin-Samos, L. Optical Properties of Saturated and Unsaturated Carbonyl Defects in Polyethylene. J. Phys. Chem. B 2018, 122, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Easteal, A.J.; Chen, X.D. Ethylene and oxygen permeability through polyethylene packaging films. Packag. Technol. Sci. 1998, 11, 169–178. [Google Scholar] [CrossRef]
- Rogers, C.E. Polymers Permeability. In Permeation of Gases and Vapours in Polymers; Chapman & Hall: London, UK, 1985; Chapter 2; pp. 11–73. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef] [Green Version]
- Berland, K.; Jiao, Y.; Lee, J.H.; Rangel, T.; Neaton, J.B.; Hyldgaard, P. Assessment of two hybrid van der Waals density functionals for covalent and non-covalent binding of molecules. J. Chem. Phys. 2017, 146, 234106. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.R. Handbook of Bond Dissociation Energies in Organic Compounds By Yu-Ran Luo (University of South Florida, St. Petersburg); CRC Press LLC: Boca Raton, FL, USA, 2003; pp. xii + 380. ISBN 0-8493-1589-1. [Google Scholar]
- Bongiorno, A.; Pasquarello, A. Oxygen Diffusion through the Disordered Oxide Network during Silicon Oxidation. Phys. Rev. Lett. 2002, 88, 125901. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J. Flow of gases through polyethylene. J. Polym. Sci. 1961, 50, 413–439. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1961.1205015412 (accessed on 21 June 2021). [CrossRef]
- Vaghjiani, G.L.; Ravishankara, A.R. Kinetics and mechanism of OH reaction with CH sub 3 OOH. J. Phys. Chem. 1989, 93. [Google Scholar] [CrossRef]
- Gugumus, F. Thermolysis of polyethylene hydroperoxides in the melt: 1. Experimental kinetics of hydroperoxide decomposition. Polym. Degrad. Stab. 2000, 69, 23–34. [Google Scholar] [CrossRef]
- Vereecken, L.; Nguyen, T.L.; Hermans, I.; Peeters, J. Computational study of the stability of α-hydroperoxyl- or α-alkylperoxyl substituted alkyl radicals. Chem. Phys. Lett. 2004, 393, 432–436. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the thermal oxidation reactions of polymers 2. Thermal oxidation of polyethylene. Polym. Degrad. Stab. 2002, 76, 329–340. [Google Scholar] [CrossRef]
- Shimada, S.; Hori, Y.; Kashiwabara, H. Free radicals trapped in polyethylene matrix: 2. Decay in single crystals and diffusion. Polymer 1977, 18, 25–31. [Google Scholar] [CrossRef]
- Shimada, S.; Hori, Y.; Kashiwabara, H. Relation between diffusion controlled decay of radicals and α-relaxation in polyethylene and polyoxymethylene. Polymer 1981, 22, 1377–1384. [Google Scholar] [CrossRef]
- Zolotova, N.V.; Denisov, E.T. Mechanism of propagation and degenerate chain branching in the oxidation of polypropylene and polyethylene. J. Polym. Sci. Part A 1 Polym. Chem. 1971, 9, 3311–3320. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/pol.1971.150091117 (accessed on 21 June 2021). [CrossRef]
- Gugumus, F. Physico-chemical aspects of polyethylene processing in an open mixer. Part 15: Product yields on bimolecular hydroperoxide decomposition. Polym. Degrad. Stab. 2005, 89, 517–526. [Google Scholar] [CrossRef]
- Nangia, P.S.; Benson, S.W. The kinetics of the interaction of peroxy radicals. II. Primary and secondary alkyl peroxy. Int. J. Chem. Kinet. 1980. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1002/kin.550120105 (accessed on 21 June 2021). [CrossRef]
- Hickel, B. Absorption spectra and kinetics of methyl and ethyl radicals in water. J. Phys. Chem. 1975, 79, 1054–1059. [Google Scholar] [CrossRef]
- Rudakov, E.; Volkova, L.K.; Tretyakov, V.P. Low selectivity of reactions of OH radicals with alkanes in acqueous solutions. React. Kinet. Catal. Lett. 1981, 16, 333–337. [Google Scholar] [CrossRef]
- Petrøuj, J.; Marchal, J. Mechanism of ketone formation in the thermooxidation and radiolytic oxidation of low density polyethylene. Radiat. Phys. Chem. 1980, 16, 27–36. [Google Scholar] [CrossRef]
- Gugumus, F. Physico-chemical aspects of polyethylene processing in an open mixer. Part 16: Mechanisms and kinetics of ketone formation at low temperature. Polym. Degrad. Stab. 2005, 90, 53–66. [Google Scholar] [CrossRef]















| Reaction | Activation Energy [eV] | ||
|---|---|---|---|
| Description | Label in Figure 2 | Molecule | Crystal |
| oxygen capture | 2 | no barrier | no barrier |
| –H abstraction | 3a | 0.84 | 0.82 |
| –H abstraction | 3b | 1.37 | 1.41 |
| –H abstraction | 3c | 1.71 | 1.54 |
| bimolecular H-abstraction | 4 | – | 0.72 |
| unimolecular PO–OH bond cleavage | 5 | 2.09 | – |
| pseudo-unimolec. POOH decomposition | 6 | – | 1.02 |
| bimolecular POOH disproportionation | 7 | – | 1.54 |
| bimolecular alkoxy/peroxy reaction | 8 | – | 0.2 |
| POOH decomposition by OH (1) | 9a | no barrier | no barrier |
| POOH decomposition by OH (2) | 9b | no barrier | no barrier |
| unimolecular POOH decomposition | 9c | 1.73 (average) | 2.06 |
| POOH decomposition by POO | 10 | – | 0.63 |
| POOH decomposition by P | 11 | – | 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Colin, X.; Roma, G. Atomic Scale Mechanisms Controlling the Oxidation of Polyethylene: A First Principles Study. Polymers 2021, 13, 2143. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132143
Ahn Y, Colin X, Roma G. Atomic Scale Mechanisms Controlling the Oxidation of Polyethylene: A First Principles Study. Polymers. 2021; 13(13):2143. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132143
Chicago/Turabian StyleAhn, Yunho, Xavier Colin, and Guido Roma. 2021. "Atomic Scale Mechanisms Controlling the Oxidation of Polyethylene: A First Principles Study" Polymers 13, no. 13: 2143. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132143