Design and Self-Assembling Behaviour of Calamitic Reactive Mesogens with Lateral Methyl and Methoxy Substituents and Vinyl Terminal Group
Abstract
:1. Introduction
2. Materials and Methods
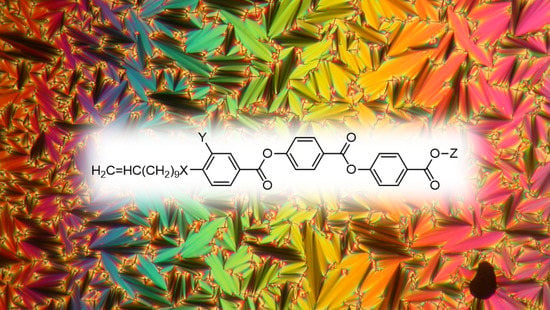
2.1. Design and Synthesis
2.2. Experimental Methods and Techniques
3. Results and Discussion
3.1. Mesomorphic Behaviour
3.2. Structural Properties
4. Summary of the Results and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lagerwall, J.P.F.; Scalia, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Nakayama, M.; Kajiyama, S.; Kumamoto, A.; Nishimura, T.; Ikuhara, Y.; Yamato, M.; Kato, T. Stimuli-responsive hydroxyapatite liquid crystal with macroscopically controllable ordering and magneto-optical functions. Nat. Commun. 2018, 9, 568. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Shibaev, V.P.; Bobrovsky, A.Y. Liquid crystalline polymers: Development trends and photocontrollable materials. Russ. Chem. Rev. 2017, 86, 1024–1072. [Google Scholar] [CrossRef]
- Hughes, T.; Simon, G.P.; Saito, K. Chemistries and capabilities of photo-formable and photoreversible crosslinked polymer networks. Mater. Horizons 2019, 6, 1762–1773. [Google Scholar] [CrossRef]
- Dierking, I. Polymer Network Stabilized Liquid Crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Rudquist, P.; Elfström, D.; Lagerwall, S.T.; Dąbrowski, R. Polymer-Stabilized Orthoconic Antiferroelectric Liquid Crystals. Ferroelectrics 2006, 344, 177–188. [Google Scholar] [CrossRef]
- Czerwiński, M.; Urbańska, M.; Bennis, N.; Rudquist, P. Influence of the type of phase sequence and polymer-stabilization on the physicochemical and electro-optical properties of novel high-tilt antiferroelectric liquid crystalline materials. J. Mol. Liq. 2019, 288, 111057. [Google Scholar] [CrossRef]
- Czerwiński, M.; de Blas, M.G.; Bennis, N.; Herman, J.; Dmochowska, E.; Otón, J.M. Polymer stabilized highly tilted antiferroelectric liquid crystals—The influence of monomer structure and phase sequence of base mixtures. J. Mol. Liq. 2021, 327, 114869. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Svyakhovskiy, S.; Bogdanov, A.; Shibaev, V.; Cigl, M.; Hamplová, V.; Bubnov, A. Photocontrollable Photonic Crystals Based on Porous Silicon Filled with Photochromic Liquid Crystalline Mixture. Adv. Opt. Mater. 2020, 8, 2001267. [Google Scholar] [CrossRef]
- Rešetič, A.; Milavec, J.; Domenici, V.; Zupančič, B.; Bubnov, A.; Zalar, B. Stress-strain and thermomechanical characterization of nematic to smectic A transition in a strongly-crosslinked bimesogenic liquid crystal elastomer. Polymer 2018, 158, 96–102. [Google Scholar] [CrossRef]
- Domenici, V.; Milavec, J.; Bubnov, A.; Pociecha, D.; Župančič, B.; Rešetič, A.; Hamplová, V.; Górecka, E.; Zalar, B. Effect of co-monomers’ relative concentration on self-assembling behaviour of side-chain liquid crystalline elastomers. RSC Adv. 2014, 4, 44056–44064. [Google Scholar] [CrossRef]
- Milavec, J.; Rešetič, A.; Bubnov, A.; Zalar, B.; Domenici, V. Dynamic investigations of liquid crystalline elastomers and their constituents by 2H NMR spectroscopy. Liq. Cryst. 2018, 45, 2158–2173. [Google Scholar] [CrossRef]
- Rešetič, A.; Milavec, J.; Zupančič, B.; Domenici, V.; Zalar, B. Polymer-dispersed liquid crystal elastomers. Nat. Commun. 2016, 7, 13140. [Google Scholar] [CrossRef]
- Bubnov, A.; Domenici, V.; Hamplova, V.; Kašpar, M.; Zalar, B. First liquid single crystal elastomer containing lactic acid derivative as chiral co-monomer: Synthesis and properties. Polymer 2011, 52, 4490–4497. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Bubnov, A.; Hamplova, V.; Kašpar, M.; Glogarová, M. Effect of Molecular Structure on Chiro-Optical and Photo-Optical Properties of Smart Liquid Crystalline Polyacrylates. Macromolecules 2013, 46, 4276–4284. [Google Scholar] [CrossRef]
- Bubnov, A.; Cigl, M.; Machado, A.M.; Pociecha, D.; Hamplova, V.; Cidade, M.T. Design of calamitic self-assembling reactive mesogenic units: Mesomorphic behaviour and rheological characterisation. Liq. Cryst. 2017, 45, 561–573. [Google Scholar] [CrossRef]
- Colen, J.; Han, M.; Zhang, R.; Redford, S.A.; Lemma, L.M.; Morgan, L.; Ruijgrok, P.V.; Adkins, R.; Bryant, Z.; Dogic, Z.; et al. Machine learning active-nematic hydrodynamics. Proc. Natl. Acad. Sci. USA 2021, 118, 2016708118. [Google Scholar] [CrossRef]
- Kárný, M.; Guy, T.V. On support of imperfect Bayesian participants. In Decision Making with Imperfect Decision Makers; Intelligent Systems Reference Library; Springer: Berlin/Heidelberg, Germany, 2012; Volume 28, pp. 29–56. [Google Scholar] [CrossRef]
- Kárný, M.; Guy, T.V. Preference Elicitation within Framework of Fully Probabilistic Design of Decision Strategies. IFAC-PapersOnLine 2019, 52, 239–244. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Piryazev, A.; Anokhin, D.V.; Ivanov, D.; Sinitsyna, O.; Hamplova, V.; Kaspar, M.; Bubnov, A. Photo-Orientation Phenomena in Photochromic Liquid Crystalline Azobenzene-Containing Polymethacrylates with Different Spacer Length. Macromol. Chem. Phys. 2017, 218, 1700127. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Cigl, M.; Hamplova, V.; Pociecha, D.; Bubnov, A. Azobenzene-containing LC polymethacrylates highly photosensitive in broad spectral range. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2962–2970. [Google Scholar] [CrossRef]
- Tóth-Katona, T.; Cigl, M.; Fodor-Csorba, K.; Hamplova, V.; Jánossy, I.; Kašpar, M.; Vojtylová, T.; Hampl, F.; Bubnov, A. Functional Photochromic Methylhydrosiloxane-Based Side-Chain Liquid-Crystalline Polymers. Macromol. Chem. Phys. 2014, 215, 742–752. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Cigl, M.; Hamplová, V.; Dorovatovskii, P.; Ostrovskii, B.; Bubnov, A. The effect of spacer and alkyl tail lengths on the photoorientation processes in amorphousized films of azobenzene-containing liquid crystalline polymethacrylates. Liq. Cryst. 2019, 47, 377–383. [Google Scholar] [CrossRef]
- Domenici, V.; Milavec, J.; Župančič, B.; Bubnov, A.; Hamplová, V.; Zalar, B. Brief overview on2H NMR studies of polysiloxane-based side-chain nematic elastomers. Magn. Reson. Chem. 2014, 52, 649–655. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, W.; Gao, Y.; Zhang, C.; Yang, H. Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships. Polymers 2018, 10, 794. [Google Scholar] [CrossRef] [Green Version]
- Bubnov, A.; Kašpar, M.; Hamplová, V.; Glogarová, M.; Samaritani, S.; Galli, G.; Andersson, G.; Komitov, L. Polar liquid crystalline monomers with two or three lactate groups for the preparation of side chain polysiloxanes. Liq. Cryst. 2006, 33, 559–566. [Google Scholar] [CrossRef]
- Petrova, I.; Gaj, A.; Pochiecha, D.; Shcherbina, M.A.; Makarova, N.N.; Bubnov, A. Design and self-assembling behaviour of comb-like stereoregular cyclolinear methylsiloxane copolymers with chiral lactate groups. Liq. Cryst. 2019, 46, 25–36. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Jiang, Z.; Wang, Z. Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating. Polymers 2020, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Honnef, K. Optical and Thermomechanical Properties of Doped Polyfunctional Acrylate Copolymers. Polymers 2018, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Kizhakidathazhath, R.; Nishikawa, H.; Okumura, Y.; Higuchi, H.; Kikuchi, H. High-Performance Polymer Dispersed Liquid Crystal Enabled by Uniquely Designed Acrylate Monomer. Polymers 2020, 12, 1625. [Google Scholar] [CrossRef]
- Dmochowska, E.; Herman, J.; Czerwiński, M.; Stulov, S.; Bubnov, A.; Kula, P. Self-assembling behaviour of chiral calamitic monoacrylates targeted for polymer stabilisation of polar smectic phases in chiral liquid crystals. J. Mol. Liq. 2021, 331, 115723. [Google Scholar] [CrossRef]
- Herman, J.; Dmochowska, E.; Czerwiński, M. Synthesis of new chiral mono- and diacrylates for ferro- and antiferroelectric liquid crystals. J. Mol. Liq. 2018, 271, 353–360. [Google Scholar] [CrossRef]
- Bubnov, A.; Cigl, M.; Sedláčková, N.; Pociecha, D.; Böhmová, Z.; Hamplová, V. Self-assembling behaviour of new functional photosensitive cinnamoyl-based reactive mesogens. Liq. Cryst. 2020, 47, 2276–2291. [Google Scholar] [CrossRef]
- Kašpar, M.; Bubnov, A.; Sedláková, Z.; Stojanović, M.; Havlicek, J.; Obadović, D.Ž.; Ilavský, M. Liquid crystalline polybutadiene diols with chiral thiol side-chain units. Eur. Polym. J. 2008, 44, 233–243. [Google Scholar] [CrossRef]
- Cidade, M.T.; Pereira, G.; Bubnov, A.; Hamplová, V.; Kaspar, M.; Casquilho, J. Rheological characterisation of a liquid-crystalline diol and its dependence with an applied electric field. Liq. Cryst. 2012, 39, 191–197. [Google Scholar] [CrossRef]
- Kašpar, M.; Bubnov, A.; Hamplova, V.; Novotna, V.; Lhotáková, I.; Havlicek, J.; Ilavský, M. Synthesis and Mesomorphic Properties of New Chiral Liquid-Crystalline Diols. Mol. Cryst. Liq. Cryst. 2005, 428, 49–63. [Google Scholar] [CrossRef]
- Cigl, M.; Bubnov, A.; Kašpar, M.; Hampl, F.; Hamplova, V.; Pacherová, O.; Svoboda, J. Photosensitive chiral self-assembling materials: Significant effects of small lateral substituents. J. Mater. Chem. C 2016, 4, 5326–5333. [Google Scholar] [CrossRef]
- Kaspar, M.; Hamplová, V.; Pakhomov, S.A.; Stibor, I.; Sverenyák, H.; Bubnov, A.; Glogarová, M.; Vanek, P. The effect of a lateral substituent on the mesomorphic properties in a series of ferroelectric liquid crystals with a 2-alkoxypropionate unit. Liq. Cryst. 1997, 22, 557–561. [Google Scholar] [CrossRef]
- Bubnov, A.; Kašpar, M.; Novotna, V.; Hamplova, V.; Glogarová, M.; Kapernaum, N.; Giesselmann, F. Effect of lateral methoxy substitution on mesomorphic and structural properties of ferroelectric liquid crystals. Liq. Cryst. 2008, 35, 1329–1337. [Google Scholar] [CrossRef]
- Stojanović, M.; Bubnov, A.; Obadović, D.Ž.; Hamplova, V.; Cvetinov, M.; Kašpar, M. Effect of a bulky lateral substitution by chlorine atom and methoxy group on self-assembling properties of lactic acid derivatives. Mater. Chem. Phys. 2014, 146, 18–25. [Google Scholar] [CrossRef]
- Pytlarczyk, M.; Dmochowska, E.; Czerwiński, M.; Herman, J. Effect of lateral substitution by chlorine and fluorine atoms of 4-alkyl-p-terphenyls on mesomorphic behaviour. J. Mol. Liq. 2019, 292, 111379. [Google Scholar] [CrossRef]
- Kašpar, M.; Bubnov, A.; Hamplova, V.; Málková, Z.; Pirkl, S.; Glogarová, M. Effect of lateral substitution by fluorine and bromine atoms in ferroelectric liquid crystalline materials containing a 2-alkoxypropanoate unit. Liq. Cryst. 2007, 34, 1185–1192. [Google Scholar] [CrossRef]
- Żurowska, M.U.; Dziaduszek, J.; Szala, M.; Morawiak, P.; Bubnov, A. Effect of lateral fluorine substitution far from the chiral center on mesomorphic behaviour of highly titled antiferroelectric (S) and (R) enantiomers. J. Mol. Liq. 2018, 267, 504–510. [Google Scholar] [CrossRef]
- Milewska, K.; Drzewiński, W.; Czerwiński, M.; Dabrowski, R. Design, synthesis and mesomorphic properties of chiral benzoates and fluorobenzoates with direct SmCA*-Iso phase transition. Liq. Cryst. 2015, 42, 1601–1611. [Google Scholar] [CrossRef]
- Węgłowska, D.; Czerwiński, M.; Kula, P.; Mrukiewicz, M.; Mazur, R.; Herman, J. Fast-response halogenated 4-alkyl-4′′-cyano-p-terphenyls as dual frequency addressing nematics. Fluid Phase Equilibria 2020, 522, 112770. [Google Scholar] [CrossRef]
- Czerwiński, M.; Gaładyk, K.; Morawiak, P.; Piecek, W.; Chrunik, M.; Kurp, K.; Kula, P.; Jaroszewicz, L.R. Pyrimidine-based ferroelectric mixtures—The influence of oligophenyl based chiral doping system. J. Mol. Liq. 2020, 303, 112693. [Google Scholar] [CrossRef]
- Kašpar, M.; Bílková, P.; Bubnov, A.; Hamplova, V.; Novotna, V.; Glogarová, M.; Knížek, K.; Pociecha, D. New chlorine-substituted liquid crystals possessing frustrated TGBA and SmQ phases. Liq. Cryst. 2008, 35, 641–651. [Google Scholar] [CrossRef]
- Podoliak, N.; Cigl, M.; Hamplová, V.; Pociecha, D.; Novotná, V. Multichiral liquid crystals based on terphenyl core laterally substituted by chlorine atom. J. Mol. Liq. 2021, 336, 116267. [Google Scholar] [CrossRef]
- Podoliak, N.; Hamplová, V.; Kašpar, M.; Novotná, V.; Glogarová, M.; Pociecha, D.; Górecka, E. Highly tilted smectogens with bromine-substituted molecular core. Liq. Cryst. 2013, 40, 321–328. [Google Scholar] [CrossRef]
- Deptuch, A.; Jaworska-Gołąb, T.; Marzec, M.; Urbańska, M.; Tykarska, M. Cold crystallization from chiral smectic phase. Phase Transit. 2018, 92, 126–134. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F.; Radcliffe, M.D. Optical and x-ray evidence of the “de Vries”Sm−A*–Sm−C*transition in a non-layer-shrinkage ferroelectric liquid crystal with very weak interlayer tilt correlation. Phys. Rev. E 2002, 66, 031703. [Google Scholar] [CrossRef]
- Shibaev, V.P. Liquid-crystalline polymer systems: From the past to the present. Polym. Sci. Ser. A 2014, 56, 727–762. [Google Scholar] [CrossRef]
- Sulyanov, S.N.; Dorovatovskii, P.V.; Bobrovsky, A.Y.; Shibaev, V.P.; Cigl, M.; Hamplova, V.; Bubnov, A.; Ostrovskii, B.I. Mesomorphic and structural properties of liquid crystalline side-chain polymethacrylates: From smectic C* to columnar phases. Liq. Cryst. 2018, 46, 825–834. [Google Scholar] [CrossRef]
- Huang, H.-M.; Chuang, E.-Y.; Chen, F.-L.; Lin, J.-D.; Hsiao, Y.-C. Color-Indicating, Label-Free, Dye-Doped Liquid Crystal Organic-Polymer-Based-Bioinspired Sensor for Biomolecule Immunodetection. Polymers 2020, 12, 2294. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Z.; Xu, M.; Lu, H. Tunable liquid crystal microlens array with negative and positive optical powers based on a self-assembled polymer convex array. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, K.-H.; Kim, H.-R.; Kim, J.-H. Dynamic focusing microlens array using liquid crystalline polymer and a liquid crystal. Proc. SPIE Int. Soc. Opt. Eng. 2006, 6352, 63521H. [Google Scholar] [CrossRef]
- Shen, Z.; Tang, M.; Chen, P.; Zhou, S.; Ge, S.; Duan, W.; Wei, T.; Liang, X.; Hu, W.; Lu, Y. Planar Terahertz Photonics Mediated by Liquid Crystal Polymers. Adv. Opt. Mater. 2020, 8, 1902124. [Google Scholar] [CrossRef]







| Mesogen | m.p. | c.p. | pH | T/ΔH | PH | T/ΔH | PH | T/ΔH | pH | T/ΔH | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UKHG | 101.3 (+72.2) | 129.4 (+0.9) | Cr | 67.8 (−63.2) | SmC | 73.0 (−0.02) | SmA | 90.5 (–0.02) | N | 128.7 (−1.1) | Iso |
| UKHM | 37.7 (+1.8) | 37.7 (+1.8) | Cr | 36.3 (−2.2) | - | - | - | Iso | |||
| UVHG | 109.4 (+83.2) | 109.4 (+83.2) | Cr | 70.5 (−61.9) | - | SmA | 91.7 (–0.4) | N | 105.1 (−1.3) | Iso | |
| UVHGET | 103.5 (+60.1) | 125.1 (+0.9) | Cr | 31.5 (−17.5) | - | SmA | 91.9 (–0.1) | N | 124.2 (−1.0) | Iso | |
| UTHH8 | 95.4 (+90.2) | 141.5 (+8.0) | Cr | 65.1 (−75.2) | SmC | 113.7 (−0.1) | SmA | 138.4 (–8.4) | - | Iso |
| Mesogen Short Name and the Cartoon of the Most Extended Molecule | L (Å) |
|---|---|
UVHG | 42.4 Å |
UKHG | 42.6 Å |
UKHM | 42.6 Å |
UVHGET | 37.7 Å |
UTHH8 | 42.2 Å |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubnov, A.; Cigl, M.; Penkov, D.; Otruba, M.; Pociecha, D.; Chen, H.-H.; Hamplová, V. Design and Self-Assembling Behaviour of Calamitic Reactive Mesogens with Lateral Methyl and Methoxy Substituents and Vinyl Terminal Group. Polymers 2021, 13, 2156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132156
Bubnov A, Cigl M, Penkov D, Otruba M, Pociecha D, Chen H-H, Hamplová V. Design and Self-Assembling Behaviour of Calamitic Reactive Mesogens with Lateral Methyl and Methoxy Substituents and Vinyl Terminal Group. Polymers. 2021; 13(13):2156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132156
Chicago/Turabian StyleBubnov, Alexej, Martin Cigl, Deyvid Penkov, Marek Otruba, Damian Pociecha, Hsiu-Hui Chen, and Věra Hamplová. 2021. "Design and Self-Assembling Behaviour of Calamitic Reactive Mesogens with Lateral Methyl and Methoxy Substituents and Vinyl Terminal Group" Polymers 13, no. 13: 2156. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132156