Strength and Biocompatibility of Heparin-Based Calcium Phosphate Cement Grafted with Ferulic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CHA, F-CHA, and CPC + CHA Composites
2.3. Strength, Injection, and Dispersion Tests
2.4. In Vitro Measurements
2.4.1. Cytotoxicity toward L929 Cells
2.4.2. Attachment, Proliferation, and Mineralization of D1 Osteoprogenitor Cell Cultures on Sample Surfaces
Cell Attachment and Morphological Observation
Semi-Quantitative Detection of Alkaline Phosphatase (ALP) Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification of CHA
3.1.1. XRD Phase Identification
3.1.2. FTIR Spectral Analysis
3.1.3. TEM Images and CHA Nanorod Aspect Ratios
3.1.4. Cytotoxicity of CHA
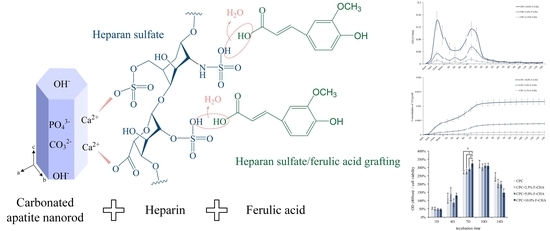
3.2. Identification of F-CHA and the Corresponding Templating Mechanism
3.3. Release Characteristics, Strength, Phase, Morphology, and Injectability of CPC + F-CHA
3.3.1. Individual Time and Cumulative Release Characteristics of Ferulic Acid
3.3.2. Compressive and Diametral Tensile Strengths of CPC + F-CHA Composites
3.3.3. SEM Images of the Fracture Surfaces and XRD Phase Identification of CPC + F-CHA Composites after Immersion for 1 Day
3.3.4. Injection and Disintegration Tests of the CPC + F-CHA Composites in ddH2O
3.4. Cytotoxicity toward L929, Cell Attachment, Proliferation, and Differentiation of D1 Cultured with CPC + F-CHA
3.4.1. Cell Viability and Morphologies of Sample Extract Culture with L929 Cells
3.4.2. Morphology and ALP Activity of D1 Progenitor Bone Cells on Various CPC + F-CHA Composite Surfaces
4. Conclusions
- L25K is a suitable mediator for impregnating bone, promoting ferulic acid, and compositing with CPC bone cement.
- The release process of CPC + F-CHA composites shows three stages, including an early burst release from the composite surface, followed by the diffusion release of ferulic acid within the composites, and, finally, the slow release of the encapsulated drug. These findings prove that F-CHA may be an effective drug carrier.
- The addition of F-CHA did not affect apatite formation. Increases in the addition of F-CHA resulted in a decrease in the strength of the product obtained. The CS of the CPC + 2.5, 5.0% F-CHA 2.5%, and 5% additive groups were approximately 50 MPa; thus, these materials may be used as load-bearing devices. The group added with CPC + 10%F-CHA achieved a CS of only 30 MPa; while this strength is relatively low, the obtained material may still find applications in non-load bearing orthopedic restoration.
- After 1 h of culture, D1 cells cultured in the CPC-only group retained their spherical shape, but the cell morphology of cells in all other experimental CPC + F-CHA groups was flat. These results demonstrate that ferulic acid can promote progenitor bone cell attachment. On the 10th day of D1 cell culture, the amount of ALP production in the CPC + 10.0%F-CHA group was significantly higher than that in other treatment groups. This finding indicates that the addition of F-CHA in CPC increases the ability of a single cell to secrete ALP.
- It can be seen that the controlled amount of heparin can prepare uniform nanorod-shaped CHA with good biocompatibility. The grafting of ferulic acid to CPC yielded a material that could be used as a slow-release drug carrier, thereby increasing the applicability of CPC in orthopedic tissue engineering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melville, A.J.; Harrison, J.; Gross, K.A.; Forsythe, J.S.; Trounson, A.O.; Mollard, R. Mouse embryonic stem cell colonisation of carbonated apatite surfaces. Biomaterials 2006, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Frank-Kamenetskaya, O.; Kol’tsov, A.; Kuz’mina, M.; Zorina, M.; Poritskaya, L. Ion substitutions and non-stoichiometry of carbonated apatite-(CaOH) synthesised by precipitation and hydrothermal methods. J. Mol. Struct. 2011, 992, 9–18. [Google Scholar] [CrossRef]
- Haung, S.-M.; Chen, J.-C.; Chang, K.-C.; Ko, C.-L.; Lin, D.-J.; Chen, W.-C. Synthesis of nanorod apatites with templates at critical micelle concentrations and in vitro evaluation of cytotoxicity and antimicrobial activity. J. Asian Ceram. Soc. 2021, 1–12. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; He, D.; Liu, F.; Liu, R. Preparation and characterization of hydroxyapatite/chondroitin sulfate composites by biomimetic synthesis. Mater. Chem. Phys. 2008, 112, 838–843. [Google Scholar] [CrossRef]
- El-Hamshary, H.; El-Naggar, M.E.; El-Faham, A.; Abu-Saied, M.A.; Ahmed, M.K.; Al-Sahly, M. Preparation and Characterization of Nanofibrous Scaffolds of Ag/Vanadate Hydroxyapatite Encapsulated into Polycaprolactone: Morphology, Mechanical, and In Vitro Cells Adhesion. Polymers 2021, 13, 1327. [Google Scholar] [CrossRef]
- Sadeghinia, A.; Davaran, S.; Salehi, R.; Jamalpoor, Z. Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combination of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells. Biomed. Pharmacother. 2019, 109, 1924–1931. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Arul, K.T.; Chandra, V.S.; Manikandan, E.; Kalkura, S.N. Agarose encapsulated mesoporous carbonated hydroxyapatite nanocomposites powder for drug delivery. J. Photochem. Photobiol. B Biol. 2017, 166, 220–231. [Google Scholar] [CrossRef]
- Du, K.; Li, Z.; Fang, X.; Cao, T.; Xu, Y. Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia. Eur. J. Cell Biol. 2017, 96, 496–503. [Google Scholar] [CrossRef]
- Folwarczna, J.; Zych, M.; Burczyk, J.; Trzeciak, H.; Trzeciak, H. Effects of Natural Phenolic Acids on the Skeletal System of Ovariectomized Rats. Planta Med. 2009, 75, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.E.; Xu, H.H.; Simon, C.G.; Takagi, S.; Chow, L.C.; Simonjr, C. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials 2005, 26, 5002–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, C.-L.; Chen, J.-C.; Tien, Y.-C.; Hung, C.-C.; Wang, J.-C.; Chen, W.-C. Osteoregenerative capacities of dicalcium phosphate-rich calcium phosphate bone cement. J. Biomed. Mater. Res. A 2014, 103, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Zhang, Y. Premixed tricalcium silicate/sodium phosphate dibasic cements for root canal filling. Mater. Chem. Phys. 2021, 257, 123682. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Taheri, M.M.; Kadir, M.R.A.; Shokuhfar, T.; Hamlekhan, A.; Shirdar, M.R.; Naghizadeh, F. Fluoridated hydroxyapatite nanorods as novel fillers for improving mechanical properties of dental composite: Synthesis and application. Mater. Des. 2015, 82, 119–125. [Google Scholar] [CrossRef]
- Chen, W.-C.; Cheng, I.-T.; Chang, K.-C.; Haung, S.-M.; Chen, J.-C.; Shih, C.-J. Heparin as a biomimetic template on nanoapatite rods with tunable aspect ratio: Synthesis and biocompatibility. J. Aust. Ceram. Soc. 2021, 57, 825–834. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lin, J.-H.C.; Ju, C.-P. Transmission electron microscopic study on setting mechanism of tetracalcium phosphate/dicalcium phosphate anhydrous-based calcium phosphate cement. J. Biomed. Mater. Res. 2003, 64, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Ko, C.-L.; Hung, C.-C.; Tyan, Y.-C.; Lai, C.-H.; Chen, W.-C.; Wang, C.-K. Deriving fast setting properties of tetracalcium phosphate/dicalcium phosphate anhydrous bone cement with nanocrystallites on the reactant surfaces. J. Dent. 2010, 38, 158–165. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, Y.; Chen, X.; Zhu, P.; Wei, S. Biomimetic synthesis and biocompatibility evaluation of carbonated apatites template-mediated by heparin. Mater. Sci. Eng. C 2013, 33, 2905–2913. [Google Scholar] [CrossRef]
- Ternent, L.; Mayoh, D.; Lees, M.; Davies, G.-L. Heparin-stabilised iron oxide for MR applications: A relaxometric study. J. Mater. Chem. B 2016, 4, 3065–3074. [Google Scholar] [CrossRef] [Green Version]
- Lafon, J.P.; Champion, E.; Bernache-Assollant, D. Processing of AB-type carbonated hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)y ceramics with controlled composition. J. Eur. Ceram. Soc. 2008, 28, 139–147. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Li, H.; Liu, J.; Xie, Y.; Wang, J.; Zhang, Y. Preparation, bioactivity and mechanism of nano-hydroxyapatite/sodium alginate/chitosan bone repair material. J. Appl. Biomater. Funct. Mater. 2018, 16, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2012, 87, 1037–1052. [Google Scholar] [CrossRef]
- Khairoun, I.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Effect of calcium carbonate on clinical compliance of apatitic calcium phosphate bone cement. J. Biomed. Mater. Res. 1997, 38, 356–360. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Fang, X.; Li, Z. Ferulic acid suppresses interleukin-1β-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1α signaling pathway. Immun. Inflamm. Dis. 2021. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chen, C.-H.; Chang, K.-C.; Liu, S.-M.; Ko, C.-L.; Shih, C.-J.; Sun, Y.-S.; Chen, W.-C. Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo. Polymers 2021, 13, 2081. [Google Scholar] [CrossRef]
- Liang, J.; Li, P.; Wang, Q.; Liao, S.; Hu, W.; Zhao, Z.; Li, Z.; Yin, B.; Mao, N.; Ding, L.; et al. Ferulic acid promotes bone defect repair after radiation by maintaining the stemness of skeletal stem cells. Stem Cells Transl. Med. 2021. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.S.; Sabbieti, M.G.; Agas, D.; Marchetti, L.; Panero, S. Polysaccharides immobilized in polypyrrole matrices are able to induce osteogenic differentiation in mouse mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2012, 8, 989–999. [Google Scholar] [CrossRef] [PubMed]















| Designated Groups | Nomenclature |
|---|---|
| Comparative processes without heparin | Control CHA nanorods |
| Templated by heparin LEO INJ 25,000 unit/mL | L25K |
| Templated by heparin LEO INJ 50,000 unit/mL | L50K |
| Templated by heparin LEO INJ 100,000 unit/mL | L100K |
| Using L25K templated CHA for ferulic acid impregnation and composite CPC with a ratio of 2.5 wt% L25K/CPC | CPC + 2.5% F-CHA |
| Using L25K templated CHA for ferulic acid impregnation and composite CPC with a ratio of 5.0 wt% L25K/CPC | CPC + 5.0% F-CHA |
| Using L25K templated CHA for ferulic acid impregnation and composite CPC with a ratio of 10.0 wt% L25K/CPC | CPC + 10.0% F-CHA |
| Dimensions and Aspect Ratio | Control CHA Nanorods Mean (S.D.) a | L25K Mean (S.D.) a | L50K Mean (S.D.) a | L100K Mean (S.D.) a |
|---|---|---|---|---|
| Length (nm) | 70.08 (28.36) | 70.32 (20.94) | 60.62 (21.04) | 52.76 (8.71) |
| Width (nm) | 24.35 (6.16) | 19.84 (5.20) | 17.51 (3.69) | 14.93 (6.16) |
| Aspect length-to-width ratio | 2.88 | 3.54 | 3.46 | 3.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-C.; Chen, J.-C.; Cheng, I.-T.; Haung, S.-M.; Liu, S.-M.; Ko, C.-L.; Sun, Y.-S.; Shih, C.-J.; Chen, W.-C. Strength and Biocompatibility of Heparin-Based Calcium Phosphate Cement Grafted with Ferulic Acid. Polymers 2021, 13, 2219. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132219
Chang K-C, Chen J-C, Cheng I-T, Haung S-M, Liu S-M, Ko C-L, Sun Y-S, Shih C-J, Chen W-C. Strength and Biocompatibility of Heparin-Based Calcium Phosphate Cement Grafted with Ferulic Acid. Polymers. 2021; 13(13):2219. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132219
Chicago/Turabian StyleChang, Kai-Chi, Jian-Chih Chen, I-Tse Cheng, Ssu-Meng Haung, Shih-Ming Liu, Chia-Ling Ko, Ying-Sui Sun, Chi-Jen Shih, and Wen-Cheng Chen. 2021. "Strength and Biocompatibility of Heparin-Based Calcium Phosphate Cement Grafted with Ferulic Acid" Polymers 13, no. 13: 2219. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132219