Composite Anion Exchange Membranes Fabricated by Coating and UV Crosslinking of Low-Cost Precursors Tested in a Redox Flow Battery
Abstract
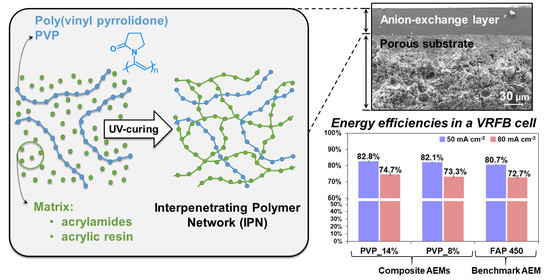
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Scanning Electron Microscopy (SEM)
2.4. Water Uptake
2.5. Area-Specific Resistance
2.6. Permeability to Vanadium Cations
2.7. VRFB Single-Cell Performance Test
2.8. Self-Discharge Experiment
2.9. Chemical Stability in VO2+ Solution
3. Results
3.1. Ion Exchange Coating Formulation and Fabrication
3.2. Area-Specific Resistance
3.3. Permeability to Vanadium Ions
3.4. VRFB Single Cell Performance
3.5. Stability in Vanadium VO2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Alkaline direct alcohol fuel cells using an anion exchange membrane. J. Power Sources 2005, 150, 27–31. [Google Scholar] [CrossRef]
- Li, H.; Zou, L. Ion-exchange membrane capacitive deionization: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Gubler, L. Membranes and separators for redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Düerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes 2021, 11, 214. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.; Zhao, Y.; Zhang, H. Mechanism of Polysulfone-Based Anion Exchange Membranes Degradation in Vanadium Flow Battery. ACS Appl. Mater. Interfaces 2015, 7, 19446–19454. [Google Scholar] [CrossRef]
- Wedege, K.; Dražević, E.; Konya, D.; Bentien, A. Organic Redox Species in Aqueous Flow Batteries: Redox Potentials, Chemical Stability and Solubility. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage 2018, 15, 010801. [Google Scholar] [CrossRef]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef] [Green Version]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium Electrolyte Studies for the Vanadium Redox Battery—A Review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhou, X.L.; An, L.; Wei, L.; Zhao, T.S. A high-performance flow-field structured iron-chromium redox flow battery. J. Power Sources 2016, 324, 738–744. [Google Scholar] [CrossRef]
- Leung, P.K.; Ponce-De-León, C.; Low, C.T.J.; Shah, A.A.; Walsh, F.C. Characterization of a zinc-cerium flow battery. J. Power Sources 2011, 196, 5174–5185. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced Hydrogen-Bromine Flow Batteries with Improved Efficiency, Durability and Cost. J. Electrochem. Soc. 2016, 163, A5049–A5056. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Geysens, P.; Li, Y.; Vankelecom, I.; Fransaer, J.; Binnemans, K. Highly soluble 1,4-diaminoanthraquinone derivative for nonaqueous symmetric redox flow batteries. ACS Sustain. Chem. Eng. 2020, 8, 3832–3843. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kasherman, D.; Hong, D.R.; Kazacos, M. Characteristics and performance of 1 kW UNSW vanadium redox battery. J. Power Sources 1991, 35, 399–404. [Google Scholar] [CrossRef]
- Okada, T.; Xie, G.; Gorseth, O.; Kjelstrup, S.; Nakamura, N.; Arimura, T. Ion and water transport characteristics of Nafion membranes as electrolytes. Electrochim. Acta 1998, 43, 3741–3747. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Tong, L.; Yu, L. Facial expression recognition based on PHOG feature and sparse representation. In Proceedings of the 2016 35th Chinese Control Conference (CCC), Chengdu, China, 27–29 July 2016; pp. 3869–3874. [Google Scholar] [CrossRef]
- Page, K.A.; Rowe, B.W. An overview of polymer electrolyte membranes for fuel cell applications. ACS Symp. Ser. 2012, 1096, 147–164. [Google Scholar] [CrossRef]
- Mohammadi, T.; Skyllas Kazacos, M. Evaluation of the chemical stability of some membranes in vanadium solution. J. Appl. Electrochem. 1997, 27, 153–160. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A multilayered membrane for vanadium redox flow battery. J. Power Sources 2012, 203, 190–194. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; He, Z.; Zhou, Z. A novel branched side-chain-type sulfonated polyimide membrane with flexible sulfoalkyl pendants and trifluoromethyl groups for vanadium redox flow batteries. J. Power Sources 2017, 347, 114–126. [Google Scholar] [CrossRef]
- Wang, Z.; Ni, H.; Zhang, M.; Zhao, C.; Na, H. Preparation and characterization of sulfonated poly(arylene ether ketone ketone sulfone)s for ion exchange membranes. Desalination 2009, 242, 236–244. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Wang, T.; Xie, X.; Zhou, X.; Ye, Y. A polysulfone-based anion exchange membrane for phosphoric acid concentration and purification by electro-electrodialysis. J. Membr. Sci. 2018, 552, 86–94. [Google Scholar] [CrossRef]
- Roh, S.H.; Lim, M.H.; Sadhasivam, T.; Jung, H.Y. Investigation on physico-chemical and electrochemical performance of poly(phenylene oxide)-based anion exchange membrane for vanadium redox flow battery systems. Electrochim. Acta 2019, 325, 134944. [Google Scholar] [CrossRef]
- Tas, S.; Zoetebier, B.; Hempenius, M.A.; Vancso, G.J.; Nijmeijer, K. Monovalent cation selective crown ether containing poly(arylene ether ketone)/SPEEK blend membranes. RSC Adv. 2016, 6, 55635–55642. [Google Scholar] [CrossRef] [Green Version]
- Park, S.G.; Kwak, N.S.; Hwang, C.W.; Park, H.M.; Hwang, T.S. Synthesis and characteristics of aminated vinylbenzyl chloride-co-styrene-co-hydroxyethyl acrylate anion-exchange membrane for redox flow battery applications. J. Membr. Sci. 2012, 423, 429–437. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X.; Pan, J.; Yang, S.; Han, B.; Xue, L.; Shen, J.; Gao, C.; der Bruggen, B. Van A novel UV-crosslinked sulphonated polysulfone cation exchange membrane with improved dimensional stability for electrodialysis. Desalination 2017, 415, 29–39. [Google Scholar] [CrossRef]
- Komkova, E.N.; Stamatialis, D.F.; Strathmann, H.; Wessling, M. Anion-exchange membranes containing diamines: Preparation and stability in alkaline solution. J. Membr. Sci. 2004, 244, 25–34. [Google Scholar] [CrossRef]
- Cha, M.S.; Jeong, H.Y.; Shin, H.Y.; Hong, S.H.; Kim, T.H.; Oh, S.G.; Lee, J.Y.; Hong, Y.T. Crosslinked anion exchange membranes with primary diamine-based crosslinkers for vanadium redox flow battery application. J. Power Sources 2017, 363, 78–86. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.H.; Kim, Y.; Moon, S.H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Viswanathan, V.; Crawford, A.; Stephenson, D.; Kim, S.; Wang, W.; Li, B.; Coffey, G.; Thomsen, E.; Graff, G.; Balducci, P.; et al. Cost and performance model for redox flow batteries. J. Power Sources 2014, 247, 1040–1051. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Mizutani, Y.; Nishimura, M. Microstructure of cation exchange membranes prepared by the paste method. J. Appl. Polym. Sci. 1980, 25, 2925–2934. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Pokhidnia, E.V.; Pourcelly, G.; Nikonenko, V.V. Can the electrochemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes? J. Membr. Sci. 2018, 566, 54–68. [Google Scholar] [CrossRef]
- Gubler, L.; Vonlanthen, D.; Schneider, A.; Oldenburg, F.J. Composite Membranes Containing a Porous Separator and a Polybenzimidazole Thin Film for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2020, 167, 100502. [Google Scholar] [CrossRef]
- Chieng, S.C.; Kazacos, M.; Skyllas-Kazacos, M. Preparation and evaluation of composite membrane for vanadium redox battery applications. J. Power Sources 1992, 39, 11–19. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, Z.; Huang, L.; Wang, C.; Zhao, Y.; Fu, Q.; Zheng, A.; Zhang, H.; Li, X. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Sprenkle, V.; Wang, W. Polyvinyl Chloride/Silica Nanoporous Composite Separator for All-Vanadium Redox Flow Battery Applications. J. Electrochem. Soc. 2013, 160, A1215–A1218. [Google Scholar] [CrossRef]
- Sanz, L.; Lloyd, D.; Magdalena, E.; Palma, J.; Kontturi, K. Description and performance of a novel aqueous all-copper redox flow battery. J. Power Sources 2014, 268, 121–128. [Google Scholar] [CrossRef]
- Wei, X.; Li, B.; Wang, W. Porous polymeric composite separators for redox flow batteries. Polym. Rev. 2015, 55, 247–272. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kim, J.H.; Jho, J.Y.; Won, J.; Kang, Y.S. Influence of the addition of PVP on the morphology of asymmetric polyimide phase inversion membranes: Effect of PVP molecular weight. J. Membr. Sci. 2004, 236, 203–207. [Google Scholar] [CrossRef]
- Han, M.J.; Nam, S.T. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Membr. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Wu, C.; Lu, S.; Wang, H.; Xu, X.; Peng, S.; Tan, Q.; Xiang, Y. A novel polysulfone-polyvinylpyrrolidone membrane with superior proton-to-vanadium ion selectivity for vanadium redox flow batteries. J. Mater. Chem. A 2015, 4, 1174–1179. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Wei, X.; Li, F.; Zhang, M.; Zhang, J.; Chen, J.; Wang, R. Highly selective sulfonated poly(ether ether ketone)/polyvinylpyrrolidone hybrid membranes for vanadium redox flow batteries. J. Mater. Sci. 2020, 55, 16822–16835. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Zeng, Y.K.; Zhang, Z.H. Polyvinylpyrrolidone-based semi-interpenetrating polymer networks as highly selective and chemically stable membranes for all vanadium redox flow batteries. J. Power Sources 2016, 327, 374–383. [Google Scholar] [CrossRef]
- Zhao, N.; Riley, H.; Song, C.; Jiang, Z.; Tsay, K.-C.; Neagu, R.; Shi, Z. Ex-Situ Evaluation of Commercial Polymer Membranes for Vanadium Redox Flow Batteries (VRFBs). Polymers 2021, 13, 926. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, D. Ultra-low vanadium ion permeable electrolyte membrane for vanadium redox flow battery by pore filling of PTFE substrate. Energy Storage Mater. 2020, 31, 105–114. [Google Scholar] [CrossRef]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of anion-exchange blend membranes on vanadium redox flow batteries. Membranes 2019, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Alagiri, K.; Prabhu, K.R. Efficient synthesis of carbonyl compounds: Oxidation of azides and alcohols catalyzed by vanadium pentoxide in water using tert-butylhydroperoxide. Tetrahedron 2011, 67, 8544–8551. [Google Scholar] [CrossRef]
- Shaabani, A.; Mirzaei, P.; Naderi, S.; Lee, D.G. Green oxidations. The use of potassium permanganate supported on manganese dioxide. Tetrahedron 2004, 60, 11415–11420. [Google Scholar] [CrossRef]
- Rawalay, S.S.; Shechter, H. Oxidation of primary, secondary, and tertiary amines with neutral permanganate. Simple method for degrading amines to aldehydes and ketones. J. Org. Chem. 1967, 32, 3129–3131. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yang, B. Chemical transformations of quaternary ammonium salts: Via C-N bond cleavage. Org. Biomol. Chem. 2020, 18, 1057–1072. [Google Scholar] [CrossRef]








| Name | Acronym | Supplier |
|---|---|---|
| Hexafunctional polyester acrylate oligomer Ebecryl 830 | EBE830 | Allnex B.V. |
| N,N′-Methylenebis(acrylamide) | MBAAM | Merck (Sigma Aldrich GmbH) |
| N-[3-(Dimethylamino)propyl]metha-crylamide | DMEA | Merck (Sigma Aldrich GmbH) |
| N-Hydroxyethylacrylamide | HEAA | Merck (Sigma Aldrich GmbH) |
| Poly(vinyl pyrrolidone) | PVP | BTC Chemical Distribution GmbH |
| Irgacure 500 (50% 1-Hydroxy-cyclohexyl-phenyl-ketone, 50% Benzophenone) | I500 | Ciba Specialty Chemicals Inc. |
| PVP [g] | DMEA [g] | HEAA [g] | MBAAM [g] | EBE830 [g] | I500 [g] | |
|---|---|---|---|---|---|---|
| PVP_6% | 0.85 | 6.3 | 3 | 0.75 | 2.2 | 0.4 |
| PVP_8% | 1.1 | 6.3 | 3 | 0.75 | 2.2 | 0.4 |
| PVP_11% | 1.6 | 6.2 | 3 | 0.75 | 2.2 | 0.4 |
| PVP_14% | 2.1 | 6.3 | 3 | 0.75 | 2.3 | 0.4 |
| PVP_16% | 2.5 | 6.3 | 3 | 0.75 | 2.4 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charyton, M.; Deboli, F.; Fischer, P.; Henrion, G.; Etienne, M.; Donten, M.L. Composite Anion Exchange Membranes Fabricated by Coating and UV Crosslinking of Low-Cost Precursors Tested in a Redox Flow Battery. Polymers 2021, 13, 2396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152396
Charyton M, Deboli F, Fischer P, Henrion G, Etienne M, Donten ML. Composite Anion Exchange Membranes Fabricated by Coating and UV Crosslinking of Low-Cost Precursors Tested in a Redox Flow Battery. Polymers. 2021; 13(15):2396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152396
Chicago/Turabian StyleCharyton, Martyna, Francesco Deboli, Peter Fischer, Gerard Henrion, Mathieu Etienne, and Mateusz L. Donten. 2021. "Composite Anion Exchange Membranes Fabricated by Coating and UV Crosslinking of Low-Cost Precursors Tested in a Redox Flow Battery" Polymers 13, no. 15: 2396. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152396