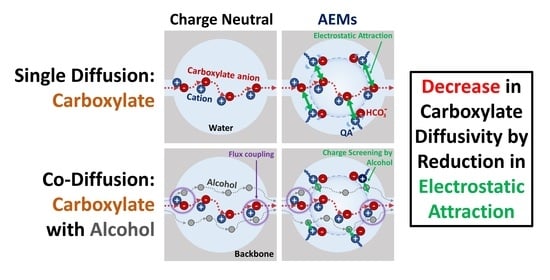
Transport and Co-Transport of Carboxylate Ions and Ethanol in Anion Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEGDA and PEGDA-APTA Membranes
2.3. Counterion Conversion via Ion Exchange from Cl− to HCO3−
2.4. Ionic Conductivity Measurement
2.5. Ion Exchange Capacity
2.6. Water Content, Density and Water Volume Fraction
2.7. Diffusion Cell Experiment
2.8. Sorption–Desorption Experiment
2.9. Diffusivity Calculation and Estimation
3. Results
3.1. Water Uptake, Density and Water Volume Fraction
3.2. Counterion Conversion, Ionic Conductivity and IEC
3.3. Permeation
3.4. Sorption
3.5. Diffusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion Exchange Membranes: Current Status and Moving Forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Geise, G.; Hickner, M.; Logan, B. Ionic Resistance and Permselectivity Tradeoffs in Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Yamazaki, S.; Ioroi, T.; Senoh, H.; Yasuda, K. Direct Ethanol Fuel Cells Using an Anion Exchange Membrane. J. Power Sources 2008, 185, 621–626. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, D.; Lee, Y.N.; Yoon, Y.S.; Kim, D.-J. Solid Solution Palladium-Nickel Bimetallic Anode Catalysts by Co-Sputtering for Direct Urea Fuel Cells (DUFC). J. Power Sources 2019, 431, 259–264. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wei, K.; Hassanvand, A.; Freeman, B.D.; Kentish, S.E. Single and Binary Ion Sorption Equilibria of Monovalent and Divalent Ions in Commercial Ion Exchange Membranes. Water Res. 2020, 175, 115681. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly Quaternized Polystyrene Ionomers for High Performance Anion Exchange Membrane Water Electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Gabardo, C.M.; Reyes, A.; O’Brien, C.P.; Holdcroft, S.; Pintauro, P.; Bahar, B.; Hickner, M.; Bae, C.; Sinton, D.; et al. Designing Anion Exchange Membranes for CO2 Electrolysers. Nat. Energy 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Carter, B.M.; Dobyns, B.M.; Beckingham, B.S.; Miller, D.J. Multicomponent Transport of Alcohols in an Anion Exchange Membrane Measured by In-Situ ATR FTIR Spectroscopy. Polymer 2017, 123, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Carter, B.M.; Keller, L.; Wessling, M.; Miller, D.J. Preparation and Characterization of Crosslinked Poly(Vinylimidazolium) Anion Exchange Membranes for Artificial Photosynthesis. J. Mater. Chem. A 2019, 7, 23818–23829. [Google Scholar] [CrossRef]
- Krödel, M.; Carter, B.M.; Rall, D.; Lohaus, J.; Wessling, M.; Miller, D.J. Rational Design of Ion Exchange Membrane Material Properties Limits the Crossover of CO2 Reduction Products in Artificial Photosynthesis Devices. ACS Appl. Mater. Interfaces 2020, 12, 12030–12042. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Bell, A.T. Design of an Artificial Photosynthetic System for Production of Alcohols in High Concentration from CO2. Energy Environ. Sci. 2015, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Houle, F.A. Chapter 10: Membranes for Solar Fuels Devices. In Integrated Solar Fuel Generators; Energy and Environment Series; RCS: London, UK, 2018; pp. 341–385. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C 2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.-C.; Bell, A.T.; Weber, A.Z. Towards Membrane-Electrode Assembly Systems for CO2 Reduction: A Modeling Study. Energy Environ. Sci. 2019, 12, 1950–1968. [Google Scholar] [CrossRef] [Green Version]
- Amel, A.; Gavish, N.; Zhu, L.; Dekel, D.R.; Hickner, M.A.; Ein-Eli, Y. Bicarbonate and Chloride Anion Transport in Anion Exchange Membranes. J. Membr. Sci. 2016, 514, 125–134. [Google Scholar] [CrossRef]
- Dischinger, S.M.; Gupta, S.; Carter, B.M.; Miller, D.J. Transport of Neutral and Charged Solutes in Imidazolium-Functionalized Poly (Phenylene Oxide) Membranes for Artificial Photosynthesis. Ind. Eng. Chem. Res. 2019, 59, 5257–5266. [Google Scholar] [CrossRef] [Green Version]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Effect of Fixed Charge Group Concentration on Equilibrium Ion Sorption in Ion Exchange Membranes. J. Mater. Chem. A 2017, 5, 4638–4650. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Ion Diffusion Coefficients in Ion Exchange Membranes: Significance of Counterion Condensation. Macromolecules 2018, 51, 5519–5529. [Google Scholar] [CrossRef]
- Kamcev, J.; Galizia, M.; Benedetti, F.M.; Jang, E.-S.; Paul, D.R.; Freeman, B.D.; Manning, G.S. Partitioning of Mobile Ions between Ion Exchange Polymers and Aqueous Salt Solutions: Importance of Counter-Ion Condensation. Phys. Chem. Chem. Phys. 2016, 18, 6021–6031. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Ion Activity Coefficients in Ion Exchange Polymers: Applicability of Manning’s Counterion Condensation Theory. Macromolecules 2015, 48, 8011–8024. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Geise, G.M.; Falcon, L.P.; Freeman, B.D.; Paul, D.R. Sodium Chloride Sorption in Sulfonated Polymers for Membrane Applications. J. Membr. Sci. 2012, 423, 195–208. [Google Scholar] [CrossRef]
- Geise, G.M.; Freeman, B.D.; Paul, D.R. Sodium Chloride Diffusion in Sulfonated Polymers for Membrane Applications. J. Membr. Sci. 2013, 427, 186–196. [Google Scholar] [CrossRef]
- Geise, G.M.; Doherty, C.M.; Hill, A.J.; Freeman, B.D.; Paul, D.R. Free Volume Characterization of Sulfonated Styrenic Pentablock Copolymers Using Positron Annihilation Lifetime Spectroscopy. J. Membr. Sci. 2014, 453, 425–434. [Google Scholar] [CrossRef]
- Kalakkunnath, S.; Kalika, D.S.; Lin, H.; Freeman, B.D. Segmental Relaxation Characteristics of Cross-Linked Poly (Ethylene Oxide) Copolymer Networks. Macromolecules 2005, 38, 9679–9687. [Google Scholar] [CrossRef]
- Sagle, A.C.; Ju, H.; Freeman, B.D.; Sharma, M.M. PEG-Based Hydrogel Membrane Coatings. Polymer 2009, 50, 756–766. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental Water and Salt Transport Properties of Polymeric Materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Kim, J.M.; Beckingham, B.S. Comonomer Effects on Co-Permeation of Methanol and Acetate in Cation Exchange Membranes. Eur. Polym. J. 2021, 147, 110307. [Google Scholar] [CrossRef]
- Beckingham, B.S.; Lynd, N.A.; Miller, D.J. Monitoring Multicomponent Transport Using in Situ ATR FTIR Spectroscopy. J. Membr. Sci. 2018, 550, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Dobyns, B.M.; Zhao, R.; Beckingham, B.S. Multicomponent Transport of Methanol and Acetate in a Series of Crosslinked PEGDA-AMPS Cation Exchange Membranes. J. Membr. Sci. 2020, 614, 118486. [Google Scholar] [CrossRef]
- Allegrezza, A.E.; Parekh, B.S.; Parise, P.L.; Swiniarski, E.J.; White, J.L. Chlorine Resistant Polysulfone Reverse Osmosis Modules. Desalination 1987, 64, 285–304. [Google Scholar] [CrossRef]
- Kim, J.M.; Beckingham, B.S. Transport and Co-transport of Carboxylate Ions and Alcohols in Cation Exchange Membranes. J. Polym. Sci. 2021, 1–14. [Google Scholar] [CrossRef]
- Soltanieh, M.; Sahebdelfar, S. Interaction Effects in Multicomponent Separation by Reverse Osmosis. J. Membr. Sci. 2001, 183, 15–27. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, N.; Freeman, B.D.; Chen, C.-C. Mobile Ion Partitioning in Ion Exchange Membranes Immersed in Saline Solutions. J. Membr. Sci. 2020, 620, 118760. [Google Scholar] [CrossRef]
- Yan, N.; Paul, D.R.; Freeman, B.D. Water and Ion Sorption in a Series of Cross-Linked AMPS/PEGDA Hydrogel Membranes. Polymer 2018, 146, 196–208. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion. Exchange; Dover: Downers Grove, IL, USA, 1995; ISBN 9780486687841. [Google Scholar]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Thompson, K.A.; Mathias, R.; Kim, D.; Kim, J.; Rangnekar, N.; Johnson, J.R.; Hoy, S.J.; Bechis, I.; Tarzia, A.; Jelfs, K.E.; et al. N-Aryl-Linked Spirocyclic Polymers for Membrane Separations of Complex Hydrocarbon Mixtures. Sci. N. Y. 2020, 369, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane Technology and Applications. John Wiley & Sons, Ltd: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Geise, G.M.; Freeman, B.D.; Paul, D.R. Characterization of a Sulfonated Pentablock Copolymer for Desalination Applications. Polymer 2010, 51, 5815–5822. [Google Scholar] [CrossRef]
- Jang, E.-S.; Kamcev, J.; Kobayashi, K.; Yan, N.; Sujanani, R.; Dilenschneider, T.J.; Park, H.B.; Paul, D.R.; Freeman, B.D. Influence of Water Content on Alkali Metal Chloride Transport in Cross-Linked Poly (Ethylene Glycol) Diacrylate.1. Ion Sorption. Polymer 2019, 178, 121554. [Google Scholar] [CrossRef]
- Galizia, M.; Paul, D.R.; Freeman, B.D. Liquid Methanol Sorption, Diffusion and Permeation in Charged and Uncharged Polymers. Polymer 2016, 102, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Luo, H.; Geise, G.M. Water Content, Relative Permittivity, and Ion Sorption Properties of Polymers for Membrane Desalination. J. Membr. Sci. 2019, 574, 24–32. [Google Scholar] [CrossRef]
- Jang, E.-S.; Kamcev, J.; Kobayashi, K.; Yan, N.; Sujanani, R.; Dilenschneider, T.J.; Park, H.B.; Paul, D.R.; Freeman, B.D. Influence of Water Content on Alkali Metal Chloride Transport in Cross-Linked Poly(Ethylene Glycol) Diacrylate.2. Ion Diffusion. Polymer 2020, 192, 122316. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions II. Self-Diffusion of the Small Ions. J. Chem. Phys. 1969, 51, 934–938. [Google Scholar] [CrossRef]
- Ju, H.; Sagle, A.C.; Freeman, B.D.; Mardel, J.I.; Hill, A.J. Characterization of Sodium Chloride and Water Transport in Crosslinked Poly(Ethylene Oxide) Hydrogels. J. Membr. Sci. 2010, 358, 131–141. [Google Scholar] [CrossRef]
- Horne, W.J.; Shannon, M.S.; Bara, J.E. Correlating Fractional Free Volume to CO2 Selectivity in [Rmim][Tf2N] Ionic Liquids. J. Chem. Thermodyn. 2014, 77, 190–196. [Google Scholar] [CrossRef]
- Dobyns, B.M.; Kim, J.M.; Beckingham, B.S. Multicomponent Transport of Methanol and Sodium Acetate in Poly (Ethylene Glycol) Diacrylate Membranes of Varied Fractional Free Volume. Eur. Polym. J. 2020, 134, 109809. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. Effect of Copolymer Composition, Temperature, and Carbon Dioxide Fugacity on Pure- and Mixed-Gas Permeability in Poly(Ethylene Glycol)-Based Materials: Free Volume Interpretation. J. Membr. Sci. 2007, 291, 131–139. [Google Scholar] [CrossRef]
- Dobyns, B.M.; Kim, J.M.; Li, J.; Jiang, Z.; Beckingham, B.S. Multicomponent Transport of Alcohols in Nafion 117 Measured by in Situ ATR FTIR Spectroscopy. Polymer 2020, 209, 123046. [Google Scholar] [CrossRef]
- Caminiti, R.; Cucca, P.; Monduzzi, M.; Saba, G.; Crisponi, G. Divalent Metal-Acetate Complexes in Concentrated Aqueous Solutions. An X-ray Diffraction and NMR Spectroscopy Study. J. Chem. Phys. 1984, 81, 543–551. [Google Scholar] [CrossRef]
- Rahman, H.M.A.; Hefter, G.; Buchner, R. Hydration of Formate and Acetate Ions by Dielectric Relaxation Spectroscopy. J. Phys. Chem. B 2011, 116, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wagner, E.V.; Swinnea, J.S.; Freeman, B.D.; Pas, S.J.; Hill, A.J.; Kalakkunnath, S.; Kalika, D.S. Transport and Structural Characteristics of Crosslinked Poly(Ethylene Oxide) Rubbers. J. Membr. Sci. 2006, 276, 145–161. [Google Scholar] [CrossRef]
- Karas, F.; Hnát, J.; Paidar, M.; Schauer, J.; Bouzek, K. Determination of the Ion-Exchange Capacity of Anion-Selective Membranes. Int. J. Hydrogen Energy 2014, 39, 5054–5062. [Google Scholar] [CrossRef]
- Xua, T.; Liub, Z.; Yanga, B. Weihua Fundamental Studies of a New Series of Anion Exchange Membranes: Membrane Prepared from Poly(2,6-Dimethyl-1,4-Phenylene Oxide) (PPO) and Triethylamine. J. Membr. Sci. 2005, 249, 183–191. [Google Scholar] [CrossRef]
- Mohr, F. Lehrbuch der Chemisch-Analytischen Titrirmethode: Nach Eigenen Versuchen und Systematisch Dargestellt, 4th ed.; Druck und Verlag von Friedrich Vieweg und Sohn: Braunschweig, Germany, 1855; Volumes 1–2. [Google Scholar]
- Yasuda, H.; Lamaze, C.E.; Ikenberry, L.D. Permeability of Solutes through Hydrated Polymer Membranes. Part I. Diffusion of Sodium Chloride. Die Makromol. Chem. 1968, 118, 19–35. [Google Scholar] [CrossRef]
- Yasuda, H.; Peterlin, A.; Colton, C.K.; Smith, K.A.; Merrill, E.W. Permeability of Solutes through Hydrated Polymer Membranes. Part III. Theoretical Background for the Selectivity of Dialysis Membranes. Die Makromol. Chem. 1969, 126, 177–186. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution-Diffusion Model: A Review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Mackie, J.S.; Meares, P. The Diffusion of Electrolytes in a Cation-Exchange Resin Membrane I. Theoretical. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1955, 232, 498–509. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions, 2nd ed.; Dover Books: New York, NY, USA, 2002. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Hills, E.E.; Abraham, M.H.; Hersey, A.; Bevan, C.D. Diffusion Coefficients in Ethanol and in Water at 298K: Linear Free Energy Relationships. Fluid Phase Equilibria 2011, 303, 45–55. [Google Scholar] [CrossRef]
- Vany’sek, P. Ionic Conductivity and Diffusion at Infinite Dilution. In CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hao, L.; Leaist, D.G. Binary Mutual Diffusion Coefficients of Aqueous Alcohols. Methanol to 1-Heptanol. J. Chem. Eng. Data 1996, 41, 210–213. [Google Scholar] [CrossRef]
- Rustad, J.R.; Nelmes, S.L.; Jackson, V.E.; Dixon, D.A. Quantum-Chemical Calculations of Carbon-Isotope Fractionation in CO2 (g), Aqueous Carbonate Species, and Carbonate Minerals. J. Phys. Chem. 2008, 112, 542–555. [Google Scholar] [CrossRef]
- Tongraar, A.; Rode, B.M. The Hydration Structures of F- and Cl− Investigated by Ab Initio QM/MM Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2002, 5, 357–362. [Google Scholar] [CrossRef]
- Tongraar, A.; T-Thienprasert, J.; Rujirawat, S.; Limpijumnong, S. Structure of the Hydrated Ca2+ and Cl−: Combined X-Ray Absorption Measurements and QM/MM MD Simulations Study. Phys. Chem. Chem. Phys. 2010, 12, 10876–10887. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Sepulveda, L.; Quina, F.; Lissi, E.; Abuin, E. Binding of Electrolytes to Poly(Ethylene Oxide) in Aqueous Solutions. Macromolecules 1990, 23, 3878–3881. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient; Danish Technical Press: Copenhagen, Denmark, 1967. [Google Scholar]
- Hallinan, D.T.; Balsara, N.P. Polymer Electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Harned, H.S.; Hildreth, C.L. The Differential Diffusion Coefficients of Lithium and Sodium Chlorides in Dilute Aqueous Solution at 25°. J. Am. Chem. Soc. 1951, 73, 650–652. [Google Scholar] [CrossRef]










| APTA 1 (mol%) | PEGDA (g) | APTA (g) | Water (g) | HCPK (g) | |
|---|---|---|---|---|---|
| A0 | 0 | 8.00 | 0.00 | 2.00 | 0.008 |
| A8 | 8 | 7.80 | 0.20 | 2.00 | 0.008 |
| A12 | 12 | 7.69 | 0.31 | 2.00 | 0.008 |
| Water Uptake, (Water g/Dry Polymer g·100%) | Dry Polymer Density, (g/mL) | Water Volume Fraction, ϕw | ||||
| Cl− | HCO3− | Cl− | HCO3− | Cl− | HCO3− | |
| A0 | 68 ± 1 | 1.22 ± 0.01 | 0.45 | |||
| A8 | 70 ± 0 | 77 ± 0 | 1.24 ± 0.02 | 1.25 ± 0.05 | 0.46 | 0.49 |
| A12 | 72 ± 2 | 83 ± 1 | 1.21 ± 0.00 | 1.22 ± 0.01 | 0.46 | 0.50 |
| AMVN | 18 ± 1 | 27 ± 0 | 1.01 ± 0.00 | 1.02 ± 0.01 | 0.15 | 0.22 |
| Ionic Conductivity (σ, mS/cm) | IEC (meq/g Dry Polymer) | |||
|---|---|---|---|---|
| Cl− | HCO3− | Cl− | HCO3− | |
| A8 | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.121 | 0.125 ± 0.004 |
| A12 | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.187 | 0.190 ± 0.001 |
| AMVN | 7.0 ± 0.0 | 4.1 ± 0.0 | 1.5 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Lin, Y.-h.; Hunter, B.; Beckingham, B.S. Transport and Co-Transport of Carboxylate Ions and Ethanol in Anion Exchange Membranes. Polymers 2021, 13, 2885. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13172885
Kim JM, Lin Y-h, Hunter B, Beckingham BS. Transport and Co-Transport of Carboxylate Ions and Ethanol in Anion Exchange Membranes. Polymers. 2021; 13(17):2885. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13172885
Chicago/Turabian StyleKim, Jung Min, Yi-hung Lin, Brock Hunter, and Bryan S. Beckingham. 2021. "Transport and Co-Transport of Carboxylate Ions and Ethanol in Anion Exchange Membranes" Polymers 13, no. 17: 2885. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13172885