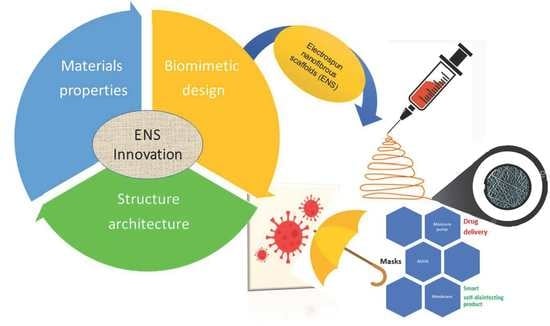
Electrospun Nanofibrous Scaffolds: Review of Current Progress in the Properties and Manufacturing Process, and Possible Applications for COVID-19
Abstract
:1. Overview of the Electrospinning Technique
1.1. State of the Art
1.2. Electrospinning Parameters
1.2.1. Solution Parameters
Concentration and/or Berry’s Number
- When the concentration is very low, micro to nano beads will be obtained. Here, electrospraying occurs instead of electrospinning, owing to the low viscosity and high surface tensions of the solution [62].
- At a specific Berry’s number and when the concentration is adequate, smooth nanofibers can be obtained.
- At high concentrations, no nanofibers are obtained; helix-shaped, coil structures, and microribbons will be observed [66].
Molecular Weight
Intrinsic Viscosity
Surface Tension
Conductivity/Surface Charge Density
1.2.2. Processing Parameters
Voltage
Flow Rate
Collectors
Distance or Spinning Height (H) between the Collector and the Tip of the Spinneret
1.2.3. Environmental Parameters
2. Potential Applications of Electrospun Nanofiber Scaffolds (ENS)
2.1. Tissue Engineering Applications
2.2. Drug or Protein Delivery
2.3. Agriculture, Food Industry, and Environment
3. Innovation in Biomimetic Design, Materials Properties, and Structure Architecture
3.1. Biomimetic Design
3.2. Materials Properties
3.3. Architecture
4. Current Progress on Elaboration Process, Implementation, and Manufacturing
6. Challenge of the Electrospun Nanofiber Scaffolds
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirjalili, M.; Zohoori, S. Review for Application of Electrospinning and Electrospun Nanofibers Technology in Textile Industry. J. Nanostruct Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Nandana, B.; Subhas, C.K. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing: Singapore, 2005. [Google Scholar]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning Over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, W.; Xia, Y.; Jiao, X.; Chen, D. Electrospun Flexible Self-Standing γ-Alumina Fibrous Membranes and Their Potential as High-Efficiency Fine Particulate Filtration Media. J. Mater. Chem. 2014, 2, 15124–15131. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Das, A.; Alzhrani, R.M.; Kang, D.; Bhaduri, S.B.; Boddu, S.H.S. Comparison of Electrospun and Solvent Cast Polylactic Acid (PLA)/Poly (vinyl alcohol) (PVA) Inserts as Potential Ocular Drug Delivery Vehicles. Mater. Sci. Eng. 2017, 77, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Zeleny, J. Instability of Electrified Liquid Surfaces. Phys. Rev. 1917, 10, 1–6. [Google Scholar] [CrossRef]
- Formhals, A. Processing and Apparatus for Preparing Artificial Threads. U.S. Patent 2077373, 10 February 1934. [Google Scholar]
- Formhals, A. Method of Producing Artificial Fiber. U.S. Patent 2158415, 16 May 1939. [Google Scholar]
- Formhals, A. Production of Artificial Fibers Forming Liquids. U.S. Patent 2323025, 29 June 1943. [Google Scholar]
- Formhals, A. Method and Apparatus for Spinning. U.S. Patent 2349950, 30 May 1944. [Google Scholar]
- Simmons, H.L. Process and apparatus for producing patterned non-woven fabrics. U.S. Patent 3280229, 18 October 1966. [Google Scholar]
- Taylor, G.I. Disintegration of Water Drops in an Electric Field. In Proceedings of the Royal Society, Series A, Mathematical, Physical & Engineering Sciences, London, UK, 28 July 1964; Volume 280, No. 1382. pp. 383–397. [Google Scholar]
- Taylor, G.I.; McEwan, A.D. Stability of a Horizontal Fluid Interface in a Vertical Electric Field. J. Fluid Mech. 1965, 22, 1–15. [Google Scholar] [CrossRef]
- Melcher, J.R.; Taylor, G.I. Electrohydrodynamics: A Review of the Role of Interfacial Shear Stresses. Annu. Rev. Fluid Mech. 1969, 1, 111–146. [Google Scholar] [CrossRef]
- Taylor, G.I. Electrically Driven Jets. In Proceedings of the Royal Society of London, Series A, Mathematical. Physical & Engineering Sciences, London, UK, 2 December 1969; Volume 313, pp. 453–475. [Google Scholar]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 7. [Google Scholar] [CrossRef]
- Haung, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. Composites Science and Technology. Health 2003, 63, 2223. [Google Scholar]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. J. Electrost. 1995, 3, 151–160. [Google Scholar] [CrossRef]
- Zussman, E.; Yarin, A.L.; Bazilevsky, A.V.; Avrahami, R.; Feldman, M. Electrospun Polyaniline/Poly(Methyl Methacrylate)-Derived Turbostratic Carbon Micro. Nanotubes 2006, 18, 348–353. [Google Scholar] [CrossRef]
- Darrell, H.R.; Alexander, L.Y. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar]
- Srikar, R.; Yarin, A.L.; Megaridis, C.M.; Bazilevsky, A.V.; Kelley, E. Desorption-Limited Mechanism of Release from Polymer Nanofibers. Langmuir 2008, 24, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Reneker, D.H. Electrospinning and the formation of nanofibers. In Structure Formation in Polymeric Fibers; Salem, D.R., Ed.; Carl Hanser Verlag: Munich, Germany, 2001; pp. 225–246. [Google Scholar]
- Liang, Y.; Ji, L.; Guo, B.; Lin, Z.; Yao, Y.F.; Li, Y.; Alcoutlabi, M.; Qiu, Y.; Zhang, X. Preparation and Electrochemical Characterization of Ionicconducting Lithium Lanthanum Titanate Oxide/Polyacrylonitrile Submicron Composite Fiber-Based Lithium-Ion Battery Separators. J. Power Sources 2011, 196, 436–441. [Google Scholar] [CrossRef]
- Schildknecht, C.E. Vinyl and Related Polymers. In Their Preparations, Properties and Applications in Rubbers, Plastics and in Medical and Industrial Arts; Wiley-Interscience: New York, NY, USA, 1952; p. 3878. [Google Scholar]
- Perepelkin, K.E.; Klyuchnikova, N.V.; Kulikova, N.A. Experimental Evaluation of Man-Made Fibre Brittleness. Fibre Chem. 1989, 21, 145–148. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A Review of Heat Treatment on Polyacrylonitrile Fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Litmanovich, A.D.; Plate, N.A. Alkaline Hydrolysis of Polyacrylonitrile; on the Reaction Mechanism. Macromol. Chem. Phys. 2000, 201, 2176–2180. [Google Scholar] [CrossRef]
- Nataraja, S.K.; Yangb, T.M. Aminabhavib, Polyacrylonitrile-Based Nanofibers-A Atate-of-the-Art Review. Prog. Polym. Sci. 2011. [Google Scholar]
- Ko, F.K.; Khan, S.; Ali, A.A.; Gogotsi, Y.; Naguib, N.; Yang, G.L.; Li, C. Structure and Properties of Carbon Nanotube Reinforced Nano-Composites. In Proceedings of the AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics and Materials Conference, Denver, CO, USA, 22–25 April 2002. [Google Scholar]
- Ali, A.A.; Geshury, A.J.; Ko, F. Ultra-Fine Carbon Fibers and Fibrous Structures From Electro-Spun PAN Polymer Solution. In Proceedings of the Fiber Society Annual Technical Conference, Natick, MA, USA, 16–18 October 2002. [Google Scholar]
- Ko, F.; Gogotsi, Y.; Ali, A.A.; Naguib, N.; Ye, H.; Yang, G.; Li, C.; Willis, P. Electrospinning of Continuous Carbon Nanotube-Filled Nanofiber Yarns. Adv. Mater. 2003, 15, 1161–1165. [Google Scholar] [CrossRef]
- Inagaki, M.; Kaneko, K.; Nishizawa, T. Nanocarbons Recent Research in Japan. Carbon 2020, 42, 1401–1417. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Review Nanofibrous Filtering Media: Filtration Problems and Solutions from Tiny Materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Mohammad, K.P.; Pirjo, H.; Ali, H. Preparation of Carbon Nanotube Embedded in PAN Nanofiber Composites by Electrospinning Process. AUTEX Res. J. 2012, 12, 1–6. [Google Scholar]
- Ali, A.A. Wet Electrospun Nanofibers. In Proceedings of the Al-Azhar Engineering Eights International Conference, Cairo, Egypt, 24–27 December 2004. [Google Scholar]
- Ali, A.A.; El-Hamid, M. Electrospinning Optimization for Precursor Carbon Nanofiber. Compos. A 2006, 37, 1681–1687. [Google Scholar] [CrossRef]
- Ali, A.A. Self-Assembled Ultra-Fine Carbon Coils by with Electrospinning. Mater. Lett. 2006, 60, 2858–2862. [Google Scholar] [CrossRef]
- Ali, A.A. Presented at Synthesis, Characterization and Industrial Applications of Nanoparticles and Nanostructure Materials Work Shop hosted by MuCSAT and NSF, New Borg El-Arab, Alexandria, Egypt, 11–15 November 2005.
- Ali, A.A.; Rutledge, G.C. Hot-Pressed Electrospun PAN Nanofibers: An Idea for Flexible Carbon Mat. J. Mater. Process. Technol. 2009, 209, 4617–4620. [Google Scholar] [CrossRef]
- Ali, A.A.; Al-Asmari, A.K. Wet-Electrospun CuNP/Carbon Nano Fiber Composites: Potential Application for Surface Mounted Component. Appl. Nanosci. 2012, 2, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Mansour, A.; Gewefel, E.; Agwa, M. Electrospun EGNPs/PS Ultra-Thin Fibril Composites: An Idea for Luminescence and Thermal Controllable Strong Fabrics. In Proceedings of the International Conference in Nanotechnology, Biotechnology and Spectroscopy (ICNBS), iSeS, Delta Pyramids Hotel, Cairo, Egypt, 29 November–1 December 2013. [Google Scholar]
- Ali, A.A. A Novel 3-D Graphite Structure from Thermally Stabilized Electrospun MWCNTs/PAN Nanofibril Composite Fabrics. Int. J. Adv. Manuf. Technol. 2014, 70, 1731–1738. [Google Scholar] [CrossRef]
- Ali, A.A.; Eldesouky, R.A.; Zoalfakar, H.S. Hot-Pressed Electrospun MWCNTs/Carbon Nano Fibril Composites: Potential Applications for Breaking Pads and Journal Bearings. In Proceedings of the 16th International AMME Conference, Cairo, Egypt, 27–29 May 2014. [Google Scholar]
- Ali, A.A.; Eldesouky, R.A.; Zoalfakar, H.S. Mechanical and Tribological Properties of Hot-Pressed Electrospun MWCNTs/Carbon Nanofibril Composite Fabrics. Int. J. Adv. Manuf. Technol. 2014, 74, 983–993. [Google Scholar] [CrossRef]
- Ali, A.A.; Eltabey, M.M.; Farouk, W.M.; Zoalfakar, H.S. Electrospun Precursor Carbon Nanofiber Optimization by Using Response Surface Methodology, I WIN EG 29–30 March 2013. J. Electrost. 2014, 72, 462–469. [Google Scholar] [CrossRef]
- Megahed, A.A.; Zoalfakar, H.S.; Hassan, A.E.A.; Ali, A.A. A Novel Polystyrene/Epoxy Ultra-Fine Fabric by Electrospinning. Polym. Adv. Technol. 2018, 29, 517–527. [Google Scholar] [CrossRef]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx Mori Silk by Electrospinning, Part 2. Process Optimization and Empirical Modeling Using Response Surface Methodology. Polymer 2004, 45, 3701. [Google Scholar] [CrossRef]
- Wächter, R.; Cordery, A. Response Surface Methodology Modeling of Diamond like Carbon Film Deposition. Carbon 1999, 37, 1529. [Google Scholar] [CrossRef]
- Hung, C.C.; Lin, H.C.; Shih, H.C. Response Surface Methodology Applied to Silicon Trench Etching in Cl2/HBr/O2 Using Transformer Coupled Plasma Technique. Solid State Electron. 2002, 46, 791. [Google Scholar] [CrossRef]
- Rosoa, M.; Lorenzettia, A.; Bescoa, S.; Montib, M.; Berti, G.; Michele, B. Modestia, Application of Empirical Modeling in Multi-layers Membrane Manufacturing. Comput. Chem. Eng. 2011, 35, 2248–2256. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Ghabrial, S.R.; Ebeid, S.J.; Serag, S.M.; Ayad, M.M. A Mathematical Modeling for Electrochemical Turning. In Proceedings of the PEDAC 5th International Conference, Bangalore, India, 26–29 October 1992; pp. 45–49. [Google Scholar]
- García-Mateos, F.; Ruiz–Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front Mater. 2019, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Jie, W.; Adelodun, A.; Kim, S.; Jo, Y. Electrospun Melamine-Blended Activated Carbon Nanofibers for Enhanced Control of Indoor CO2. J. Appl. Polym. Sci. 2019, 136, 47747. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent Advances in Carbon Nanofibers and Their Applications A Review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Wahyudiono, M.; Machmudah, S.; Kanda, H.; Okubayashi, S.; Goto, M. Formation of PVP Hollow Fibers by Electrospinning in One-Step Process at sub and Supercritical CO2. Chem. Eng. Process. Process. Intensif. 2014, 77, 1–6. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Assisted Electrospray: An Improved Micronization Process Lucia Baldino, Stefano Cardea and Ernesto Reverchon. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldino, L.; Cardea, S.; Reverchon, E. A Supercritical CO2 Assisted Electrohydrodynamic Process Used to Produce Microparticles and Microfibers of a Model Polymer. J. CO2 Util. 2019, 33, 532–540. [Google Scholar] [CrossRef]
- Deitzel, J.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Eda, G.; Shivkumar, S. Bead-to-fiber Transition in Electrospun Polystyrene. J. Appl. Polym. Sci. 2007, 106, 475–487. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D. Beaded Nanofibers Formed During Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Bang, H.; Jung, Y.; Lee, S. The Change of Bead Morphology Formed on Electrospun Polystyrene Fibers. Polymer 2003, 44, 4029–4034. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of Solvents on the Formation of Ultrathin Uniform poly (Vinyl Pyrrolidone) Nanofibers with Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of Molecular Weight on Fibrous PVA Produced by Electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, Q.B.; Lu, X.F.; Wang, C.; Wei, Y. Study on Correlation of Morphology of Electrospun Products of Polyacrylamide with Ultrahigh Molecular Weight. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2190–2195. [Google Scholar] [CrossRef]
- McKee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E.; Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; et al. Phospholipid Nonwoven Electrospun Membranes. Sciences 2006, 311, 353–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrondo, L.; St. Manley, J.R. Electrostatic Fiber Spinning from Polymer Melts, Experimental Observations on Fiber Formation and Properties. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 906–920. [Google Scholar] [CrossRef]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx Mori Silk by Electrospinning—part 1: Processing Parameters and Geometric Properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and Characterization of a Nanoscale Poly (Vinyl Alcohol) Fiber Aggregate Produced by an Electrospinning Method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of Gelatin Nanofiber Prepared from Gelatin–Formic Acid Solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, L.; Park, H.N.; Shin, S.Y.; Park, W.H.; Lee, S.C.; Kim, T.I.; Park, Y.J.; Seol, Y.J.; Lee, Y.M.; et al. Biological Efficacy of Silk Fibroin Nanofiber Membranes for Guided Bone Regeneration. J. Biotechnol. 2005, 120, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of Molecular Weight of Atactic Poly (Vinyl Alcohol) (PVA) in the Structure and Properties of PVA Nanofabric Prepared by Electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of Gelatin Fibers and Gelatin/PCL Composite Fibrous Scaffolds. J. Biomed. Mater. Res. 2004, 72, 156–165. [Google Scholar] [CrossRef]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status solidi 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(e-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on Morphology of Electrospun Poly(Vinyl Alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and Process Relationship of Electrospun Bioabsorbable Nanofiber Membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Lai, C.; Reneker, D.H.; Qiu, H.; Ye, Y.; Hou, H. Electrospun Polymer Nanofibres with Small Diameters. Nanomaterials 2006, 17, 1558–1563. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhang, Y.; Dong, C.; Sheng, J. Morphology of Ultrafine Polysulfone Fibers Prepared by Electrospinning. Polym. Int. 2004, 53, 1704–1710. [Google Scholar] [CrossRef]
- Buchko, C.J.; Chen, L.C.; Shen, Y.; Martin, D.C. Processing and Microstructural Characterization of Porous Biocompatible Protein Polymer thin Films. Polymer 1999, 40, 7397–7407. [Google Scholar] [CrossRef]
- Demir, M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Yördem, O.S.; Papila, M.; Menceloğlu, Y.Z. Effects of Electrospinning Parameters on Polyacrylonitrile Nanofiber Diameter: An Investigation by Response Surface Methodology. Mater. Des. 2008, 29, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Um, I.C.; Fang, D.; Okamoto, A.; Hsiao, B.S.; Chu, B. Formation of Water-Resistant Hyaluronic Acid Nanofibers by Blowing-Assisted Electro-Spinning and Non-Toxic Post Treatments. Polymer 2005, 46, 4853–4867. [Google Scholar] [CrossRef]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S.; Xiang, R.Z.; Chang, C.C.; Fann, W.S. Electrospinning of Continuous Aligned Polymer Fibers. Appl. Phys. Lett. 2004, 84, 1222–1224. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning Nanofibers as Uniaxially Aligned Arrays and Layer-by-Layer Stacked Films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned Biodegradable Nanofibrous Structure: A Potential Scaffold for Blood Vessel Engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Ki, C.S.; Kim, J.W.; Hyun, J.H.; Lee, K.H.; Hattori, M.; Rah, D.K.; Park, Y.H. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. Sci. 2007, 106, 3922–3928. [Google Scholar] [CrossRef]
- Mit-Uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine Electrospun Polyamide-6 Fibers: Effect of Solution Conditions on Morphology and Average Fiber Diameter. Macroml Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling Surface Morphology of Electrospun Polystyrene Fibers: Effect of Humidity and Molecular Weight in the Electrospinning Process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Abdelhady, S.S.; Zoalfakar, S.H.; Agwa, M.A.; Ali, A.A. Mechanical and Thermal Characteristics of Optimized Electrospun Nylon 6,6 Nanofibers by Using Taguchi Method. Nanofiber 2019, 14, 1950139. [Google Scholar] [CrossRef] [Green Version]
- Khang, G. Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine; Imprint Jenny Stanford Publishing: New York, NY, USA, 2017. [Google Scholar]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stems Cells Int. 2018, 2018, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Li, D.; Tao, L.; Shen, Y.; Sun, B.; Xie, X.; Ke, Q.; Mo, X.; Deng, B. Fabrication of Multilayered Nanofiber Scaffolds with a Highly Aligned Nanofiber Yarn for Anisotropic Tissue Regeneration. ACS Omega 2020, 5, 24340–24350. [Google Scholar] [CrossRef]
- Rim, N.G.; Shin, C.S.; Shin, H. Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef]
- Ma, B.; Xie, J.; Jiang, J.; Shuler, F.D.; Bartlett, D.E. Rational Design of Nanofiber Scaffolds for Orthopedic Tissue Repair and Regeneration. Nanomedicine 2013, 8, 1459–1481. [Google Scholar] [CrossRef] [Green Version]
- Xianfeng, W.; Bin, D.; Jianyong, Y.; Moran, W. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar]
- Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Bioinspired Scaffold Designs for Regenerating Musculoskeletal Tissue Interfaces. Regen. Eng. Transl. Med. 2020, 6, 451–483. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.; Singh, S. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Zikmundova, M.; Filova, E.; Mikes, P.; Jencova, V.; Kuzelova Kostakova, E.; Sinica, A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Based on Nature-Derived Polymers. In Current and Future Aspects of Nanomedicine; Khalil, I.A.H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Travis, J.; Sill, H.; von Recum, A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar]
- Romána, Z.; Dimitrios, A.L.; István, S. Recent Development of Electrospinning for Drug Delivery. Pharmaceutics 2020, 12, 5. [Google Scholar]
- Liu, M.; Zhang, Y.; Sun, S.; Abdur, R.K.; Ji, J.; Mingshi, Y.; Guangxi, Z. Recent Advances in Electrospun for Drug Delivery Purpose. J. Drug Targets 2019, 27, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit Lisa, C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 789289, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hyuk, S.Y.; Taek, G.K.; Tae, G.P. Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar]
- Bishweshwar, P.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive Electrospraying: A route to process Electrospun Scaffolds for Controlled Molecular Release. Polym Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Richa, S.; Meenakshi, B. Transdermal Drug Delivery System: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 3, 773–790. [Google Scholar]
- Rafaela, Z.C.; Meira, I.B.F.; Biscaia, C.; Nogueira, F.S.; Murakami, L.; Bernardi, S.; Paulo, R. Oliveira Solid-State Characterization and Compatibility Studies of Penciclovir, Lysine Hydrochloride, and Pharmaceutical Excipients. Materials 2019, 12, 3154. [Google Scholar]
- Karthikeyan, K.; Guhathakarta, S.; Rajaram, R.; Korrapati, P.S. Electrospun Zein/Eudragit Nanofibers Based Dual Drug Delivery System for the Simultaneous Delivery of Aceclofenac and Aantoprazole. Int. J. Pharm. 2012, 438, 1–2. [Google Scholar] [CrossRef]
- Modgill, V.; Garg, T.; Goyal, A.K.; Rath, G. Permeability Study of Ciprofloxacin from Ultra-thin Nanofibrous Film through Various Mucosal Membranes. Artif. Cells Nanomed. Biotechnol 2016, 44, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, X.; Zhang, Z.; Jing, X.; Zhang, P.; Xie, Z.G. Prevention of Local Liver Cancer Recurrence After Surgery Using Multilayered Cisplatin-Loaded Polylactide Electrospun Nanofibers. Chinese J. Polym. Sci. 2014, 32, 1111–1118. [Google Scholar] [CrossRef]
- Singh, B.; Garg, T.; Goyal, A.K.; Rath, G. Development, Optimization, and Characterization of Polymeric Electrospun Nanofiber: A New Attempt in Sublingual Delivery of Nicorandil for the Management of Angina Pectoris. Artif Cells Nanomed Biotechnol. 2016, 44, 1498–1507. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug Delivery System Based on Cyclodextrin-Naproxen Inclusion Complex Incorporated in Electrospun Polycaprolactone Nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Shen, X.-X.; Branford-White, C.; White, K.; Zhu, L.-M.; Annie Bligh, S.W. Oral Fast-Dissolving Drug Delivery Mem-branes Prepared from Electrospun Polyvinylpyrrolidone Ultrafine Fibers. Nanotechnology 2009, 20, 9. [Google Scholar] [CrossRef]
- Conn, L.; Hastings, E.; Roche, T.; Ruiz-Hernandez, E.; Schenke-Layland, K.; Walsh, C.J.; Duffy, G.P. Drug and Cell Delivery for Cardiac Regeneration. Adv. Drug Deliv. Rev. 2015, 84, 85–106. [Google Scholar]
- Kenyatta, S.W.; Chris, A.B. Delivery of Antioxidant and Anti-inflammatory Agents for Tissue Engineered Vascular Grafts. Front. Pharm. 2017, 8, 659. [Google Scholar]
- Shahriar, S.M.S.; Jagannath, M.; Mohammad, N.H.; Vishnu, R.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Teoh, S.H.; Kang, L. Polycaprolactone Scaffold as Targeted Drug Delivery System and Cell Attachment Scaffold for Postsurgical Care of Limb Salvage. Drug Deliv. Transl. Res. 2012, 2, 272–283. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive Electrospun Scaffolds Delivering Growth Factors and Genes for Tissue Engineering Applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Suk, C.J.; Sang, Y.H. Electrospun Nanofibers Surface-modified with Fluorescent Proteins. J. Bioact. Compat. Polym. 2007, 22, 508–524. [Google Scholar]
- Tıglı, R.S.; Kazaroğlu, N.M.; Mavış, B.; Gümüşderelioğlu, M. Cellular Behavior on Epidermal Growth Factor (EGF)-Immobilized PCL/Gelatin Nanofibrous Scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 207–223. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theragnostic. Adv. Mater. 2017, 29, 1603276. [Google Scholar] [CrossRef]
- Gao, H. Progress and Perspectives on Targeting Nanoparticles for Brain Drug Delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable electrospun fibers for drug delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A Review on Wound Dressings with an Emphasis on Electrospun Nanofibrous Polymeric Bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Mohammadian, F.; Eatemadi, A. Drug Loading and Delivery Using Nanofibers Scaffolds. Artificial Cells. Nanomed. Biotechnol. 2017, 45, 881–888. [Google Scholar]
- Hu, J.; Wei, J.; Liu, W.; Chen, Y. Preparation and Characterization of Electrospun PLGA/Gelatin Nanofibers as a Drug Delivery System by Emulsion Electrospinning. J. Biomater. Sci. Polym. Ed. 2013, 24, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Abdali, Z.; Liu, S.; Miller, D.W. Salinomycin-Loaded Nanofibers for Glioblastoma Therapy. Sci. Rep. 2018, 8, 9377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.; Rashid, M.H.; Arbab, A.S.; Khan, M. Encapsulation of Anticancer Drugs (5-Fluorouracil and Paclitaxel) into Polycaprolactone (PCL) Nanofibers and in Vitro Testing for Sustained and Targeted Therapy. J. Biomed. Nanotechnol. 2017, 13, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yang, L.; Xu, X.; Wang, X.; Chen, X.; Liang, Q.; Zeng, J.; Jing, X. Ultrafine Medicated Fibers Electrospun from W/O Emulsions. J. Control. Release 2005, 108, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.A.; Abou-Elkhair, R.A.I.; Parker, W.B.; Allan, P.W.; Secrist, J.A., III. 6-Methylpurine Derived Sugar Modified Nucleosides: Synthesis and Evaluation of Their Substrate Activity with Purine Nucleoside Phosphorylases. Bioorg. Chem. 2016, 65, 9–16. [Google Scholar] [CrossRef]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Aleksander Kowalewski, T.; Ding, B.; Pierini, P. Ultraviolet Light-Assisted Electrospinning of Core–Shell Fully Cross-Linked P(NIPAAm-co-NIPMAAm) Hydrogel-Based Nanofibers for Hermally Induced Drug Delivery Self-Regulation. Adv. Mater. Interfaces 2020, 7, 1–13. [Google Scholar]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Pierini, F. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun Nanofibers: New Generation Materials for Advanced Applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun Doping of Carbon Nanotubes and Platinum Nanoparticles into the β-Phase Polyvinylidene Difluoride Nanofibrous Membrane for Biosensor and Catalysis Applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Masumeh, N. Electrospun Nanofibres in Agriculture and the Food Industry: A Review. J. Sci. Food Agric. 2016, 96, 1–16. [Google Scholar]
- Castano, L.M.; Flatau, A.B. Smart Fabric Sensors and e-Textile Technologies: A Review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Hosseini, S.M.; Mohammad, Y. Application of Electrospinning Technique in Development of Intelligent Food Packaging: A short Review of Recent Trends. Food Sci. Nutr. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Prietsch, L.P.; Antunes, M.D.; Jansen-Alves, C.; Mendonça, C.R.B.; Borges, C.D.; Zavareze, E.d.R.; Zambiazi, R.C. Avocado Oil Incorporated in Ultrafine Zein Fibers by Electrospinning. Food Biophys. 2019, 14, 383–392. [Google Scholar] [CrossRef]
- Shuiliang, C.; Guanghua, H.; Alessandro, A.C.M.; Seema, A.; Greiner, A.; Haoqing, H.; Uwe, S. Electrospun Carbon Fiber Mat with Layered Architecture for Anode in Microbial Fuel Cells. Electrochem. Commun. 2011, 13, 1026–1029. [Google Scholar]
- Veluru, J.B.; Manippady, K.K.; Rajendiren, M.; Mya Mya, K.; Rayavarapu, P.R.; Appukuttan, S.N.; Seeram, R. Photocatalytic Hydrogen Generation by Splitting of Water from Electrospun Hybrid Nanostructures. Int. J. Hydrog. Energy 2013, 38, 4324–4333. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Zhang, G.; Wei, G.; Su, Z. Electrospinning Design of Functional Nanostructures for Biosensor Applications. J. Mater. Chem. B 2017, 5, 1699–1711. [Google Scholar]
- Zhang, P.; Zhao, X.; Ji, Y.; Ouyang, Z.; Wen, X.; Li, J.; Su, Z.; Wei, G. Electrospinning Graphene Quantum Dots into a Nanofibrous Membrane for Dual-Purpose Fluorescent and Electrochemical Biosensors. J. Mater. Chem. B 2015, 3, 2487–2496. [Google Scholar]
- Subhash, S. Nanofiber Electrodes for Biosensors. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Shao, P.; Yan, Z.; Chen, H.; Xiao, J. Electrospun Poly (Vinyl Alcohol)/Permutite Fibrous Film Loaded with Cinnamaldehyde for Active Food Packaging. J. Appl. Polym. Sci. 2017, 40, 46117. [Google Scholar] [CrossRef]
- Wang, K.N.; Burugapalli, W.S.J.; Halls, F.; Moussy, A.R.; Zheng, Y. Electrospun Fibro-Porou Spolyurethan Ecoating Sforimplantableglucosebiosensors. Biomaterials 2013, 34, 888–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neethirajan, S.; Ragavan, V.; Weng, X.; Chand, R. Biosensors for Sustainable Food Engineering: Challenges and Perspectives. Biosensors 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-Based (bio)Sensors for Food and Agricultural Applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Wen, P.; Wen, Y.; Zong, M.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef]
- Ashraf, A.A. New Generation of Super Absorber Nano-Fibroses Hybrid Fabric by Electro-Spinning. J. Mater. Process. Technol. 2008, 199, 193–198. [Google Scholar]
- Mohammad, M.; Hasani-Sadrabadi, I.S.; Masoud, S.; Homayoun, M. Novel Nanofiber-Based Triple-Layer Proton Exchange Membranes for Fuel Cell Applications. J. Power Sources 2011, 196, 4599–4603. [Google Scholar]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of Multilevel Structured Functional Micro-/Nanofibers and Their Applications. J. Mater. Chem. A 2013, 1, 7290. [Google Scholar] [CrossRef]
- Afeesh, R.; Nasser, A.M.; Barakat, S.; Al-Deyab, S.; Yousef, A.; Kim, H.Y. Nematic Shaped Cadmium Sulfide Doped Electrospun Nanofiber Mat: Highly Efficient, Reusable. Sol. Light Photocatal. 2012, 409, 21–29. [Google Scholar]
- Nguyen, T.D.; Kadri, O.E.; Sikavitsas, V.I.; Voronov, R.S. Scaffolds with a High Surface Area-to-Volume Ratio and Cultured Under Fast Flow Perfusion Result in Optimal O2 Delivery to the Cells in Artificial Bone Tissues. Appl. Sci. 2019, 9, 2381. [Google Scholar] [CrossRef] [Green Version]
- Darbar, D.; Reddy, M.V.; Sundarrajan, S.; Pattabiraman, R.; Ramakrishna, S.; Chowdari, B.V.R. Anodic Electrochemical Performances of MgCo2O4 Synthesized by Oxalate Decomposition Method and Electrospinning Technique for Li-ion Battery Application. Mater. Res. Bull. 2016, 73, 369–376. [Google Scholar] [CrossRef]
- Mannarino, M.M. Characterization and Modification of Electrospun Fiber Mats for Use in Composite Proton Exchange Membranes. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 2013. [Google Scholar]
- Mousavi, S.; Shahraki, F.; Aliabadi, M.; Haji, A.; Deuber, F.; Adlhart, C. Surface Enriched Nanofiber Mats for Efficient Adsorption of Cr(VI) Inspired by Mature. J. Environ. Chem. Eng. 2019, 7, 102817. [Google Scholar] [CrossRef]
- Jian, F.; Tao, N.; Tong, L.; Gai, W. Applications of Electrospun Nanofibers. Chin. Sci. Bull. 2008, 53, 2265–2286. [Google Scholar]
- Zhao, R.; Lu, X.; Wang, C. Electrospinning Based all-Nano Composite Materials: Recent Achievements and Perspectives. Compos. Commun. 2018, 10, 140–150. [Google Scholar] [CrossRef]
- Alvi, M.A.; Akhtar, M.S. An Effective and Low Cost Pd–Ce Bimetallic Decorated Carbon Nanofibers as Electro-Catalyst for Direct Methanol Fuel Cells Applications. J. Alloys Compd. 2016, 684, 524–529. [Google Scholar] [CrossRef]
- Lurie-Luke, E. Product and Technology Innovation: What Can Biomimicry Inspire? Biotechnol. Adv. 2014, 32, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Emily, B.K.; Thomas, A.M. Biomimicry: Streamlining the Front End of Innovation for Environmentally Sustainable Products. Res. Manag. 2016, 59, 40–48. [Google Scholar]
- Hayato, I.; Masato, S.; Seiji, A.; Tsutomu, K. Realistic Imitation of Mosquito’s Proboscis: Electrochemically Etched Sharp and Jagged Needles and Their Cooperative Inserting Motion. Sens. Actuators A Phys. 2011, 165, 115–123. [Google Scholar]
- Wang, X.; Ding, B.; Li, B. Biomimetic Electrospun Nanofibrous Structures for Tissue Engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Ito, Y.; Chen, X.; Kang, I.-K. Advances in Bioinspired and Biomedical Materials. ACS Symp. Ser. 2017, 1253, 153–167. [Google Scholar] [CrossRef]
- Du, M.; Gu, J.; Wang, J.; Xue, Y.; Ma, Y.; Mo, X.; Xue, S. Silk Fibroin/Poly(L-lactic Acid-co-ε-Caprolactone) Electrospun NanoFibrous Scaffolds Exert a Protective Effect Following Myocardial Infarction. Exp. Ther. Med. 2019, 17, 3989–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.; Newton, J.; Michael, I. Progess in Superhydrophobic Surface Development. Soft Matter 2008, 4, 224. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Ding, B.; Yu, J.; Sun, G.; Wang, M. Biomimicry via Electrospinning. Crit. Rev. Solid State Mater. Sci. 2012, 37, 94–114. [Google Scholar] [CrossRef]
- Yan, G.; Yu, J.; Qiu, Y.; Yi, X.; Lu, J.; Zhou, X.; Bai, X. Self-Assembly of Electrospun Polymer Nanofibers: A General Phenomenon Generating Honeycomb-Patterned Nanofibrous Structures. Langmuir 2011, 27, 4285–4289. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, X.; Yu, J.; Wang, M. Polyamide 6 Composite Nano-Fiber/net Functionalized by Polyethyleneimine on Quartz Crystal Microbalance for Highly Sensitive Formaldehyde Sensors. J. Mater. Chem. 2011, 21, 12784. [Google Scholar] [CrossRef]
- Andreas, H. Electrospun Polycaprolactone Nanofiber Scaffolds for Tissue Engineering. Ph.D. Thesis, University of Arkansas, Fayetteville, NC, USA, 2012. [Google Scholar]
- Nagam, H.S.; Rao, S. Multi-Functional Electrospun Nanofibers from Polymer Blends for Scaffold Tissue Engineering. Fibers 2019, 7, 66. [Google Scholar]
- Nemati, S.; Kim, S.; Shin, Y.M.; Shin, H. Current Progress in Application of Polymeric Nanofibers to Tissue Engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior—Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Pennel, T.; Fercana, G.; Bezuidenhout, D.; Simionescu, A.; Chuang, T.-H.; Zilla, P.; Simionescu, D. The Performance of Cross-linked Acellular Arterial Scaffolds as Vascular Grafts; Pre-Clinical Testing in Direct and Isolation Loop Circulatory Models. Biomaterials 2014, 35, 6311–6322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Bao, M.; Lou, X.; Zhou, Q.; Dong, W.; Yuan, H.; Zhang, Y. Electrospun Biomimetic Fibrous Scaffold from Shape Memory Polymer of PDLLA-co-TMC for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6, 2611–2621. [Google Scholar] [CrossRef]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguiar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef] [PubMed]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M. A Porous Hydrogel-Electrospun Composite Scaffold Made of Oxidized Alginate/Gelatin/Silk Fibroin for Tissue Engineering Application. Carbohydr. Polym. 2020, 245, 116465. [Google Scholar] [CrossRef]
- Hajiabbas, I.; Alemzadeh, M.; Vossoughi, M.; Shamloo, A. In-Situ Crosslinking of Electrospun Gelatin-Carbodiimide Nanofibers: Fabrication, Characterization, and Modeling of Solution Parameter. Chem. Eng. Commun. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Yousefzade, O.; Katsarava, R.; PuiggalÃ, J. Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 2020, 5, 49. [Google Scholar] [CrossRef]
- Dalton, P.D.; Woodfield, T.B.F.; Mironov, V.; Groll, J. Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv. Sci. 2020, 7, 1902953. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of Microbial Polysaccharide Hydrogels for Tissue Engineering and Regenerative Medicine: A Review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Teoh, J.; Ee, M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.-M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutzweiler, G.; Ndreu, H.A.; Engin, V.N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 7, 602. [Google Scholar] [CrossRef]
- Koichi, N. Chapter 1 in vitro biofabrication of Tissues and Organs, Biofabrication Micro- and Nano-Fabrication, Printing. Patterning Assem. 2013, 1–21. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Jan, H.; Dietmar, W.H. Design and Fabrication of Scaffold-Based Tissue Engineering. Bio Nano Mater. 2020, 14. [Google Scholar] [CrossRef] [Green Version]
- Ameer, P.; Kasoju, R. Strategies to Tune Electrospun Scaffold Porosity for Effective Cell Response in Tissue Engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, N.; Ivanovski, S.; Gulati, K.; Love, R.M.; Hamlet, S. Role of Offset and Gradient Architectures of 3-D Melt Electrowritten Scaffold on Differentiation and Mineralization of Osteoblasts. Biomater. Res. 2020, 24, 2. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Han, Y.; Jiang, Q.; Zhan, C.; Zhang, K.; Li, Z.; Zhang, R. Structural Design and Environmental Applications of Electrospun Nanofibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106009. [Google Scholar] [CrossRef]
- Liu, H.; Gough, C.R.; Deng, Q.; Gu, Z.; Wang, F.; Hu, X. Recent Advances in Electrospun Sustainable Composites for Biomedical, Environmental, Energy, and Packaging Applications. Int. J. Mol. Sci. 2020, 21, 4019. [Google Scholar] [CrossRef]
- Soroush, S.; Khanian, N.; Choong, T.S.Y.; Rashid, U. Recent Progress in the Design and Synthesis of Nanofibers with Diverse Synthetic Methodologies: Characterization and Potential Applications. New J. Chem. 2020, 44, 9581. [Google Scholar]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.M.; Benazzo, M.; Conti, B. The Effect of Process Parameters on Alignment of Tubular Electrospun Nanofibers for Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. Comprehensive Review Summarizing Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2015, 11, 1165–1188. [Google Scholar] [CrossRef]
- Datta, P.; Dhara, S. Engineering Porosity in Electrospun Nanofiber Sheets by Laser Engraving: A Strategy to Fabricate 3D Scaffolds for Bone Graft Applications. J. Indian. Inst. Sci. 2019, 99, 329–337. [Google Scholar] [CrossRef]
- Nestor, W.S.P.; Anton, S.; Nikita, P.; Anton, S.; Pavel, P. Direct Ink Writing Technology (3D Printing) of Graphene-Based Ceramic Nanocomposites: A Review. Nanomaterials 2020, 10, 1300. [Google Scholar]
- Sooriyaarachchi, D.; Minière, H.J.; Maharubin, S.; Tan, G.Z. Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues. Tissue Eng. Regen. Med. 2019, 16, 29–38. [Google Scholar] [CrossRef]
- Rabionet, M.; Polonio, E.; Guerra, A.; Martin, J.; Puig, T.; Ciurana, J. Design of a Scaffold Parameter Selection System with Additive Manufacturing for a Biomedical Cell Culture. Materials 2018, 11, 1427. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.S.; Wu, D.Y.; Wang, S.S. Bio-Based Polymer Nanofiber with Siliceous Sponge Spicules Prepared by Electrospinning: Preparation, Characterisation, and Functionalisation. Mater. Sci. Eng. C 2020, 108, 110506. [Google Scholar] [CrossRef]
- Rongthong, W.; Niamnont, N.; Srisuwannaket, C.; Paradee, N.; Mingvanish, W. Electrospun Gelatin Fiber Mats Mixed With C.carandas Extract and its Enhanced Stability and Bioactivity. J. Pharm. Sci. 2021, in press. [Google Scholar]
- Gupta, D.; Jassal, M.; Agrawal, A.K. Solution Properties and Electrospinning of Poly(Galacturonic Acid) Nanofibers. Carbohydr. Polym. 2019, 212, 102–111. [Google Scholar] [CrossRef]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S. Encapsulation of β-Carotene in Poly(Vinylpyrrolidone) (PVP) by Electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Eskitoros, T.Ş.M.; Bulbul, Y.E.; Dilsiz, N. Combination of Nano-Hydroxyapatite and Curcumin in a Biopolymer Blend Matrix: Characteristics and Drug Release Performance of Fibrous Composite Material Systems. Int. J. Pharm. 2020, 590, 119933. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Jia, X.W.; Liu, Q.; Kong, B.; Wang, H. Fast Dissolving Oral Films for Drug Delivery Prepared from Chitosan/Pullulan Electrospinning Nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Baek, J.; Lee, E.; Lotz, M.K.; D’Lima, D.D. Bioactive Proteins Delivery through Core-Shell Nanofibers for Meniscal Tissue Regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102090. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.E.; Petit-Breuilh, X.; Díaz, P.E.; Gacitúa, W. Manufacture of a Bio-Tissue Based on Nanocrystalline Cellulose from Chilean Bamboo Chusquea Quila and a Polymer Matrix Using Electrospinning. Nano Struct. Nano Objects 2020, 23, 100525. [Google Scholar] [CrossRef]
- Singhal, P. Preparation and Characterization of Poly (E-CAPROLACTONE) Nano Fibers by Electrospinning Technique for Tissue Enginerring Applications. Mater. Today Proc. 2021, 37, 2997–3001. [Google Scholar] [CrossRef]
- Rabionet, M.; Puig, T.; Ciurana, J. Manufacture of PCL Scaffolds through Electrospinning Technology to Accommodate Triple Negative Breast Cancer Cells Culture. Procedia CIRP 2020, 89, 98–103. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Mohammadi, Z.; Samadi, F.Y.; Isaei, E. Biocompatible Electrospinning Chitosan Nanofibers: A Novel Delivery System with Superior Local Cancer Therapy. Carbohydr. Polym. 2017, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Asadi, H.; Ghaee, A.; Nourmohammadi, J.; Mashak, A. Electrospun Zein/Graphene Oxide Nanosheet Composite Nanofibers with Controlled Drug Release as Antibacterial Wound Dressing. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 173–185. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Drobota, M.; Timpu, D.; Vasiliu, T.; Constantinescu, C.A.; Rebleanu, D.; Calin, M.; David, G. Biopolymers/poly(ε-caprolactone)/Polyethylenimine Functionalized Nano-Hydroxyapatite Hybrid Cryogel: Synthesis, Characterization and Application in gene Delivery. Mater. Sci. Eng. C 2017, 81, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-Web-Inspired PM0.3 Filters Based on Self-Sustained Electrostatic Nanostructured Networks. Adv. Mater. 2020, 32, 2002361. [Google Scholar] [CrossRef]
- Omori, Y.; Gu, T.; Bao, L.; Otani, Y.; Seto, T. Performance of Nanofiber/Microfiber Hybrid Air Filter Prepared by Wet Paper Processing. Aerosol. Sci. Technol. 2019, 53, 1149–1157. [Google Scholar] [CrossRef]
- Tabibzadeh, A.; Esghaei, M.; Soltani, S.; Yousefi, P.; Taherizadeh, M.; Safarnezhad, T.F.; Golahdooz, M.; Panahi, M.; Ajdarkosh, H.; Zamani, F.; et al. Evolutionary Study of COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) as an Emerging Coronavirus: Phylogenetic Analysis and Literature Review. Vet. Med. Sci. 2020, 00, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, C.; Zhang, Y.; Zhang, S.; Jin, M. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibodies in Pets in Wuhan, China. J. Infect. 2020, 81, 68–69. [Google Scholar] [CrossRef]
- Karunathilake, K. Positive and Negative Impacts of COVID-19, an Analysis with Special Reference to Challenges on the Supply Chain in South Asian countries. J. Soc. Econ. Dev. 2020. [Google Scholar] [CrossRef]
- Sivaraman, D.; Pradeep, P.S.; Manoharan, S.S.; Bhat, C.R.; Leela, K.V.; Venugopal, V. Current Strategies and Approaches in Combating SARS-CoV-2 Virus that Causes COVID-19. Lett. Drug Des. Discov. 2020, 17, 670–672. [Google Scholar] [CrossRef]
- Kchaou, M.; Abuhasel, K.; Khadr, M.; Hosni, F.; Alquraish, M. Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies. Appl. Sci. 2020, 10, 6040. [Google Scholar] [CrossRef]
- Global Research on Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov (accessed on 16 December 2020).
- Outlook on the Worldwide Market for Nanofibers to 2030—Initiatives Using Nanofibers to Aid the Response to COVID-19. Available online: https://www.globenewswire.com/news-release/2020/04/06/2011882/0/en/Outlook-on-the-Worldwide-Market-for-Nanofibers-to-2030-Initiatives-Using-Nanofibers-to-Aid-the-Response-to-COVID-19.html (accessed on 17 December 2020).
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun Nanofibers-Based Face Masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and Emerging Healthcare and Medicine Possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef]
- De Sio, L.; Ding, B.; Focsan, M.; Kogermann, K.; Pascoal-Faria, P.; Petronella, F.; Pierini, F. Personalized Reusable Face Masks with Smart Nano-Assisted Destruction of Pathogens for COVID-19: A Visionary Road. Chem. Eur. J. 2021, 27, 1–20. [Google Scholar]
- Zhang, Y.; Wu, L.; Wang, X.; Yu, J.; Ding, B. Super Hygroscopic Nanofibrous Membrane-Based Moisture Pump for Solar-Driven Indoor Dehumidification. Nat. Commun. 2020, 11, 3302. [Google Scholar] [CrossRef]
- Recyclable Nano-Fiber Filtered Face Masks a Boon for Supply Fiasco. Available online: https://news.kaist.ac.kr/newsen/html/news/?mode=V&mng_no=6530&skey=category&sval=research&list_s_date=&list_e_date=&GotoPage=1 (accessed on 3 January 2021).
- Khanzada, H.; Salam, A.; Qadir, M.B.; Phan, D.-N.; Hassan, T.; Munir, M.U.; Pasha, K.; Hassan, N.; Khan, M.Q.; Kim, I.S. Fabrication of Promising Antimicrobial Aloe Vera/PVA Electrospun Nanofibers for Protective Clothing. Materials 2020, 13, 3884. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Biosensors for the Detection of Bacterial and Viral Clinical Pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.G.; Liu, Y.; Shao, J. Core-Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
| Application | Polymer and Solvent Used | Product Characteristics | Ref. |
|---|---|---|---|
| Cosmetic mask | A siliceous sponge spicules (SSS) and polylactic acid (PLA). | A nanofiber composite (PLA/SSS) of 50–450-nm with enhanced thermal and mechanical properties; a slight enhancement in human foreskin fibroblast cell proliferation; a decent cytocompatibility; and antibacterial | [212] |
| A gelatin solution prepared in ethanol extracted from Crude Carissa Carandas fruits (CCE) and incorporating acetic acid. | A smooth and continuous gelatin fibers mats (GFM) with an average diameter of 235.69 ± 10.45 nm. could be obtained with the optimal conditions of 30% (w/v) gelatin solution, 25% (v/v) ethanol solution, 30% (v/v) acetic acid, a fixed electrostatic field strength of 20 kV and a 15 cm distance between spinneret tip and collector. When 15% (w/w) CCE is used, the CCE-GFM shows high DPPH radical scavenging and tyrosinase inhibitory activity. | [213] | |
| Anionic surfactants added to a natural biopolymer of galacturonic acid (PGuA) to enable its electrospinning to nanofibers. | Small spindled fibers of 2 to 10 μm length and 287 to 997 nm diameter. Large continuous fibers could be produced when an amount of 10 to 30% of high molecular weight PVA is used. | [214] | |
| Drug Delivery | A poly(vinylpyrrolidone)/PVP electrospun to encapsulate β-carotene dissolved in ethanol. | PVP/β-carotene composite nanofibers of 176 to 306 nm average diameter were able to protect the β-carotene properties | [215] |
| A blend of poly (ε-caprolactone) and poly (ethylene oxide) (PCL/PEO) incorporating a nanosized hydroxyapatite (n-HA) to carry curcumin. | A nanofiber material with slow release rate of curcumin and with a high cytotoxicity against breast cancer cell line | [216] | |
| Chitosan/pullulan carried by a shell of polylactic acid (PLA) | A nanofiber with improved thermal properties and rapid dissolving capability in water. | [217] | |
| Tissue engineering | A platelet-derived growth factor (PDGF-BB) contained within a shell of polylactic acid (PLA) and encapsulated within nanofibers | A 3D scaffolding nanofibers with microporous structure, acceptable mechanical properties and high cell compatibility. | [218] |
| Crystalline cellulose (NCC) in a matrix of cellulose acetate (CA) polymer. | a bio-tissues of nanofibers with uniform diameter, moderate thermal properties and improved mechanical properties of 30 MPa tensile strength and 1.597 MPa module of elasticity. | [219] | |
| A blend of poly(ε-caprolactone), poly(ethylene glycol), poly(ε-caprolactone) (PCEC) along with polylactide (PLA). | A biodegradable polylactide (PLA)/PCEC fibrous membranes compatible with bone tissues. | [220] | |
| Cancer therapy | A poly(ε-caprolactone) (PCL) | A scaffolding system of long nanofibers to carry breast cancer therapy | [221,222] |
| Wound dressing | A zein/Graphene oxide (GO) blend. The GO is loaded by Tetracycline hydrochloride (TCH). | A nanofiber composite with enhanced mechanical properties and improved release profile. | [223] |
| Gene delivery | A biopolymer incorporating nano-hydroxyapatite (nHAp) modified with linear polyethylenimine (LPEI), and poly(ε-caprolactone) (PCL). | A homogeneous and cohesive composite with structural characteristics, swelling and degradation behavior dependent on the size and amount of the included inorganic particles. | [224] |
| Filter media. | A poly(ε-caprolactone) (PCL) | Nanofibers (NF) of average diameters of 180 and 234 nm with improved bioprotective activity and filtration efficiency | [225,226] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kchaou, M.; Alquraish, M.; Abuhasel, K.; Abdullah, A.; Ali, A.A. Electrospun Nanofibrous Scaffolds: Review of Current Progress in the Properties and Manufacturing Process, and Possible Applications for COVID-19. Polymers 2021, 13, 916. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060916
Kchaou M, Alquraish M, Abuhasel K, Abdullah A, Ali AA. Electrospun Nanofibrous Scaffolds: Review of Current Progress in the Properties and Manufacturing Process, and Possible Applications for COVID-19. Polymers. 2021; 13(6):916. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060916
Chicago/Turabian StyleKchaou, Mohamed, Mohammed Alquraish, Khaled Abuhasel, Ahmad Abdullah, and Ashraf A. Ali. 2021. "Electrospun Nanofibrous Scaffolds: Review of Current Progress in the Properties and Manufacturing Process, and Possible Applications for COVID-19" Polymers 13, no. 6: 916. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060916