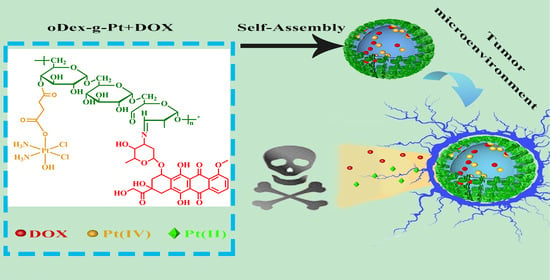
pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of Oxidized Dextran (oDex)
2.4. General Procedure for Synthesis of Platinum Conjugated oDex (oDex-g-Pt)
2.5. Conjugation of DOX with oDex-g-Pt
2.6. Preparation and Characterization of oDex-g-Pt and oDex-g-Pt+DOX NPs
2.7. pH- and Reduction-Sensitivity of oDex-g-Pt+DOX NPs
2.8. pH- and Reduction-Activated Drugs Release from oDex-g-Pt+DOX NPs
2.9. Cell Culture
2.10. In Vitro Cytotoxicity
2.11. In Vitro Cellular Uptake and Intracellular Distribution
2.12. Statistical Analysis
3. Results
3.1. Design, Synthesis and Characterization of Drug, Drug-Polymer Conjugates
3.2. Preparation and Characterization of oDex-g-Pt NPs and oDex-g-Pt+DOX NPs
3.3. Stability of oDex-g-Pt+DOX NPs
3.4. In Vitro Drugs Release
3.5. In Vitro Cytotoxicity and Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharyya, J.; Weitzhandler, I.; Ho, S.B.; McDaniel, J.R.; Li, X.; Tang, L.; Liu, J.; Dewhirst, M.; Chilkoti, A. Encapsulating a Hydrophilic Chemotherapeutic into Rod-like Nanoparticles of a Genetically Encoded Asymmetric Triblock Polypeptide Improves its Efficacy. Adv. Funct. Mater. 2017, 27, 1605421. [Google Scholar] [CrossRef]
- Cheng, C.; Sui, B.; Wang, M.; Hu, X.; Shi, S.; Xu, P. Carrier-Free Nanoassembly of Curcumin-Erlotinib Conjugate for Cancer Targeted Therapy. Adv. Healthc. Mater. 2020, 9, e2001128. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Sun, Y.; Shen, M.; Song, K.; Yin, X.; Di, W.; Duan, Y. Enhanced Chemotherapeutic Efficacy of Paclitaxel Nanoparticles Co-delivered with MicroRNA-7 by Inhibiting Paclitaxel-Induced EGFR/ERK pathway Activation for Ovarian Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7821–7831. [Google Scholar] [CrossRef]
- Park, N.H.; Cheng, W.; Lai, F.; Yang, C.; Florez de Sessions, P.; Periaswamy, B.; Wenhan Chu, C.; Bianco, S.; Liu, S.; Venkataraman, S.; et al. Addressing Drug Resistance in Cancer with Macromolecular Chemotherapeutic Agents. J. Am. Chem. Soc. 2018, 140, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Gu, Z. Bioinspired and Biomimetic Nanomedicines. Acc. Chem. Res. 2019, 52, 1255–1264. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lin, Y.; Yin, M.; Ren, J.; Qu, X. Biomineralization inspired surface engineering of nanocarriers for pH-responsive, targeted drug delivery. Biomaterials 2013, 34, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, D.; Yu, H.; Wang, M.; Liu, J.; Feng, B.; Zhou, F.; Yin, Q.; Zhang, Z.; Huang, Y.; et al. Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug-Resistant Tumor. ACS Nano 2016, 10, 3496–3508. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z.P. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials 2014, 35, 3331–3339. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, W.; Liu, J.; Dong, Z.; Liu, Z. pH-Responsive Nanoscale Covalent Organic Polymers as a Biodegradable Drug Carrier for Combined Photodynamic Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 14475–14482. [Google Scholar] [CrossRef]
- Wu, Y.; Kuang, H.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Novel hydroxyl-containing reduction-responsive pseudo-poly(aminoacid) via click polymerization as an efficient drug carrier. Polym. Chem. 2014, 5, 4488–4498. [Google Scholar] [CrossRef]
- Hartl, N.; Adams, F.; Merkel, O.M. From Adsorption to Covalent Bonding: Apolipoprotein E Functionalization of Polymeric Nanoparticles for Drug Delivery across the Blood–Brain Barrier. Adv. Therap. 2020, 4, 2000092. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Feng, J.; Wen, W.; Jia, Y.G.; Liu, S.; Guo, J. pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers 2019, 11, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Wu, S.; Hu, C.; Chen, Z.; Wang, H.; Fan, F.; Qin, Y.; Wang, C.; Sun, H.; Leng, X.; et al. Folate-targeted polymersomes loaded with both paclitaxel and doxorubicin for the combination chemotherapy of hepatocellular carcinoma. Acta Biomater. 2017, 58, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, H.; Wang, C.; Zhang, Z.; Ruan, H.; Sun, L.; Yang, C.; Li, Y.; Qin, W. Synergistic Cisplatin/Doxorubicin Combination Chemotherapy for Multidrug-Resistant Cancer via Polymeric Nanogels Targeting Delivery. ACS Appl. Mater. Interfaces 2017, 9, 9426–9436. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Wang, Z.; Ma, W.; Guo, J.; Chen, J.; Yu, Z.; Li, J.; Zhou, D. Light-Activatable Prodrug and AIEgen Copolymer Nanoparticle for Dual-Drug Monitoring and Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18691–18700. [Google Scholar] [CrossRef]
- Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum covalent shell cross-linked micelles designed to deliver doxorubicin for synergistic combination cancer therapy. Int. J. Nanomed. 2017, 12, 3697–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, H.; Yao, J.; Yu, H.; Chen, X.; Zhang, P.; Xiao, C. Glutathione-triggered dual release of doxorubicin and camptothecin for highly efficient synergistic anticancer therapy. Colloids. Surf. B Biointerfaces 2018, 169, 273–279. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Abedi, F.; Alizadeh, E.; Baradaran, B.; Annabi, N.; Akbarzadeh, A.; Davaran, S. Lysine-embedded cellulose-based nanosystem for efficient dual-delivery of chemotherapeutics in combination cancer therapy. Carbohydr. Polym. 2020, 250, 116861. [Google Scholar] [CrossRef]
- Sharma, P.K.; Taneja, S.; Singh, Y. Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan-Poly (ethylene glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [Google Scholar] [CrossRef]
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Xie, Y.; Cong, F. Reduction- and pH-Sensitive Hyaluronan Nanoparticles for Delivery of Iridium(III) Anticancer Drugs. Biomacromolecules 2017, 18, 2102–2117. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, X.; Wang, Y.; Liu, F.; Peng, J.; Zhao, X.; Yang, H.; Ma, L.; Wang, B.; Chang, C.; et al. Fabrication of Acidic pH-Cleavable Polymer for Anticancer Drug Delivery Using a Dual Functional Monomer. Biomacromolecules 2018, 19, 3874–3882. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Ke, W.; Lu, N.; Wang, Y.; Japir, A.; Mohammed, F.; Pan, Y.; Ge, Z. Glutathione and Reactive Oxygen Species Dual-Responsive Block Copolymer Prodrugs for Boosting Tumor Site-Specific Drug Release and Enhanced Antitumor Efficacy. Biomacromolecules 2020, 21, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 45, 3830–3852. [Google Scholar] [CrossRef]
- Li, D.; Su, T.; Ma, L.; Yin, F.; Xu, W.; Ding, J.; Li, Z. Dual-acidity-labile polysaccharide-di-drugs conjugate for targeted cancer chemotherapy. Eur. J. Med. Chem. 2020, 199, 112367. [Google Scholar] [CrossRef]
- Braga, C.B.; Perli, G.; Becher, T.B.; Ornelas, C. Biodegradable and pH-Responsive Acetalated Dextran (Ac-Dex) Nanoparticles for NIR Imaging and Controlled Delivery of a Platinum-Based Prodrug into Cancer Cells. Mol. Pharm. 2019, 16, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Paoli, A.; Naimo, G.D.; Mauro, L.; Amantea, D.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Self-assembling Dextran prodrug for redox- and pH-responsive co-delivery of therapeutics in cancer cells. Colloids. Surf. B Biointerfaces 2020, 185, 110537. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.; Desbrieresc, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Atanase, L. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- He, S.; Cong, Y.; Zhou, D.; Li, J.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. A dextran–platinum(IV) conjugate as a reduction-responsive carrier for triggered drug release. J. Mater. Chem. B 2015, 3, 8203–8211. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]
- Daraba, O.; Cadinoiu, A.; Rata, D.; Atanase, L.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, M.; Cheng, X.; Xu, Y.; Lv, X.; Wang, X.; Tang, R. pH/redox dual-sensitive platinum (IV)-based micelles with greatly enhanced antitumor effect for combination chemotherapy. J. Colloid Interface Sci. 2019, 541, 30–41. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J.; Tian, H.; Chen, X. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J. Control Release 2019, 295, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef] [PubMed]










| Full Name | Abbreviation |
|---|---|
| diamminedichloro-dihydroxyplatinum | DHP |
| succinic anhydride modified DHP | Pt (IV) |
| oxidized dextran | oDex |
| platinum conjugated oxidized dextran | oDex-g-Pt |
| DOX conjugated oxidized dextran | oDex-g-DOX |
| platinum plus DOX conjugated oxidized dextran | oDex-g-Pt+DOX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Wu, Y.; Xu, X.; Xu, B.; Chen, Z.; Li, T. pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation. Polymers 2021, 13, 1515. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091515
Xue X, Wu Y, Xu X, Xu B, Chen Z, Li T. pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation. Polymers. 2021; 13(9):1515. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091515
Chicago/Turabian StyleXue, Xiukun, Yanjuan Wu, Xiao Xu, Ben Xu, Zhaowei Chen, and Tianduo Li. 2021. "pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation" Polymers 13, no. 9: 1515. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091515