Charge Transport and Glassy Dynamics in Blends Based on 1-Butyl-3-vinylbenzylimidazolium Bis(trifluoromethanesulfonyl)imide Ionic Liquid and the Corresponding Polymer
Abstract
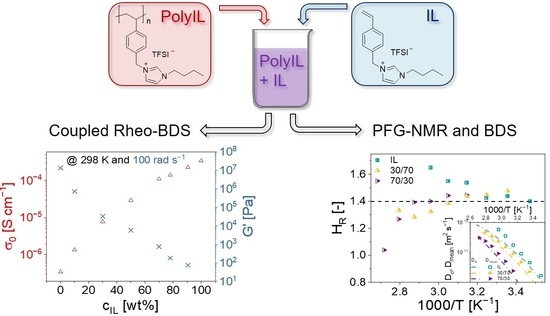
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Methods
2.3.1. Size Exclusion Chromatography (SEC)
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Wide-Angle X-ray Scattering (WAXS)
2.3.4. Rheological and Dielectric Measurements
2.3.5. Pulsed-Field Gradient Nuclear Magnetic Resonance (PFG-NMR)
3. Results and Discussion
3.1. Rheological Measurements
3.2. Dielectric Properties
3.3. Diffusion Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric ionic liquids for lithium-based rechargeable batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Ionic Liquids for Supercapacitive Energy Storage: A Mini-Review. Energy Fuels 2021, 35, 8443–8455. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 294. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2012, 1, 2719–2743. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Hoffmann, M.; Butzelaar, A.J.; Iacob, C.; Theato, P.; Wilhelm, M. Ionogels as Polymer Electrolytes for Lithium–Metal Batteries: Comparison of Poly(ethylene glycol) Diacrylate and an Imidazolium-Based Ionic Liquid Crosslinker. ACS Appl. Polym. Mater. 2022, 4, 2794–2805. [Google Scholar] [CrossRef]
- Sasabe, H.; Saito, S. Relationship between Ionic Mobility and Segmental Mobility in Polymers in the Liquid State. Polymer J. 1972, 1972, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Imrie, C.T.; Ingram, M.D. Decoupled ion transport in mesomorphic polymer electrolyte glasses. Electrochim. Acta 2001, 46, 1413–1417. [Google Scholar] [CrossRef]
- Agapov, A.L.; Sokolov, A.P. Decoupling Ionic Conductivity from Structural Relaxation: A Way to Solid Polymer Electrolytes? Macromolecules 2011, 44, 4410–4414. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Feng, H.; Fu, Y.; Cheng, S.; Carroll, B.; Kumar, R.; Novikov, V.N.; Kisliuk, A.M.; Saito, T.; Kang, N.-G.; et al. Effect of Chain Rigidity on the Decoupling of Ion Motion from Segmental Relaxation in Polymerized Ionic Liquids: Ambient and Elevated Pressure Studies. Macromolecules 2017, 50, 6710–6721. [Google Scholar] [CrossRef]
- Wei, X.; Shriver, D.F. Highly Conductive Polymer Electrolytes Containing Rigid Polymers. Chem. Mater. 1998, 10, 2307–2308. [Google Scholar] [CrossRef]
- Wang, Y.; Agapov, A.L.; Fan, F.; Hong, K.; Yu, X.; Mays, J.; Sokolov, A.P. Decoupling of ionic transport from segmental relaxation in polymer electrolytes. Phys. Rev. Lett. 2012, 108, 88303. [Google Scholar] [CrossRef]
- Iacob, C.; Sangoro, J.R.; Serghei, A.; Naumov, S.; Korth, Y.; Kärger, J.; Friedrich, C.; Kremer, F. Charge transport and glassy dynamics in imidazole-based liquids. J. Chem. Phys. 2008, 129, 234511. [Google Scholar] [CrossRef]
- Iacob, C.; Matsumoto, A.; Brennan, M.; Liu, H.; Paddison, S.J.; Urakawa, O.; Inoue, T.; Sangoro, J.; Runt, J. Polymerized Ionic Liquids: Correlation of Ionic Conductivity with Nanoscale Morphology and Counterion Volume. ACS Macro Lett. 2017, 6, 941–946. [Google Scholar] [CrossRef]
- Fan, F.; Wang, Y.; Hong, T.; Heres, M.F.; Saito, T.; Sokolov, A.P. Ion Conduction in Polymerized Ionic Liquids with Different Pendant Groups. J. Phys. Condens. Matter 2015, 48, 4461–4470. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- De Anastro, A.; Porcarelli, L.; Hilder, M.; Berlanga, C.; Galceran, M.; Howlett, P.; Forsyth, M.; Mecerreyes, D. UV-Cross-Linked Ionogels for All-Solid-State Rechargeable Sodium Batteries. ACS Appl. Energy Mater. 2019, 2, 6960–6966. [Google Scholar] [CrossRef]
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel–Polymer Electrolytes Based on Poly(Ionic Liquid)/Ionic Liquid Networks. ACS Appl. Polym. Mater. 2021, 3, 200–208. [Google Scholar] [CrossRef]
- Kataoka, T.; Ishioka, Y.; Mizuhata, M.; Minami, H.; Maruyama, T. Highly Conductive Ionic-Liquid Gels Prepared with Orthogonal Double Networks of a Low-Molecular-Weight Gelator and Cross-Linked Polymer. ACS Appl. Mater. Interfaces 2015, 7, 23346–23352. [Google Scholar] [CrossRef] [PubMed]
- Snedden, P.; Cooper, A.I.; Scott, K.; Winterton, N. Cross-Linked Polymer−Ionic Liquid Composite Materials. Macromolecules 2003, 36, 4549–4556. [Google Scholar] [CrossRef]
- Põhako-Esko, K.; Timusk, M.; Saal, K.; Lõhmus, R.; Kink, I.; Mäeorg, U. Increased conductivity of polymerized ionic liquids through the use of a nonpolymerizable ionic liquid additive. J. Mater. Res. 2013, 28, 3086–3093. [Google Scholar] [CrossRef]
- Marcilla, R.; Alcaide, F.; Sardon, H.; Pomposo, J.A.; Pozo-Gonzalo, C.; Mecerreyes, D. Tailor-made polymer electrolytes based upon ionic liquids and their application in all-plastic electrochromic devices. Electrochem. Commun. 2006, 8, 482–488. [Google Scholar] [CrossRef]
- Mogurampelly, S.; Keith, J.R.; Ganesan, V. Mechanisms Underlying Ion Transport in Polymerized Ionic Liquids. J. Am. Chem. Soc. 2017, 139, 9511–9514. [Google Scholar] [CrossRef]
- Mogurampelly, S.; Ganesan, V. Ion Transport in Polymerized Ionic Liquid–Ionic Liquid Blends. Macromolecules 2018, 51, 9471–9483. [Google Scholar] [CrossRef]
- Schuhmann, S.; Guthausen, G. NMR-Messmethoden. Chem. Unserer Zeit 2021, 55, 236–250. [Google Scholar] [CrossRef]
- Guo, X.; Wetzel, K.S.; Solleder, S.C.; Spann, S.; Meier, M.A.R.; Wilhelm, M.; Luy, B.; Guthausen, G. 1H PFG-NMR Diffusion Study on a Sequence-Defined Macromolecule: Confirming Monodispersity. Macromol. Chem. Phys. 2019, 220, 1900155. [Google Scholar] [CrossRef]
- Guo, X.; Laryea, E.; Wilhelm, M.; Luy, B.; Nirschl, H.; Guthausen, G. Diffusion in Polymer Solutions: Molecular Weight Distribution by PFG-NMR and Relation to SEC. Macromol. Chem. Phys. 2017, 218, 1600440. [Google Scholar] [CrossRef]
- Guo, X.; Theissen, S.; Claussen, J.; Hildebrand, V.; Kamphus, J.; Wilhelm, M.; Luy, B.; Guthausen, G. Dynamics of Sodium Ions and Water in Swollen Superabsorbent Hydrogels as Studied by 23Na- and 1H-NMR. Macromol. Chem. Phys. 2019, 220, 1800350. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling electrode polarization in dielectric spectroscopy: Ion mobility and mobile ion concentration of single-ion polymer electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, J.E.; Zanelotti, C.J.; Iacob, C.; Korovich, A.G.; Madsen, L.A.; Colby, R.H. Ion Transport and Mechanical Properties of Non-Crystallizable Molecular Ionic Composite Electrolytes. Macromolecules 2020, 53, 1405–1414. [Google Scholar] [CrossRef]
- Stacy, E.W.; Gainaru, C.P.; Gobet, M.; Wojnarowska, Z.; Bocharova, V.; Greenbaum, S.G.; Sokolov, A.P. Fundamental Limitations of Ionic Conductivity in Polymerized Ionic Liquids. Macromolecules 2018, 51, 8637–8645. [Google Scholar] [CrossRef]
- Gainaru, C.; Stacy, E.W.; Bocharova, V.; Gobet, M.; Holt, A.P.; Saito, T.; Greenbaum, S.; Sokolov, A.P. Mechanism of Conductivity Relaxation in Liquid and Polymeric Electrolytes: Direct Link between Conductivity and Diffusivity. J. Phys. Chem. B 2016, 120, 11074–11083. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 1. Variation of Anionic Species. J. Phys. Chem. B 2004, 108, 16593–16600. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Plancher, H.; Radosz, M.; Shen, Y. Poly(ionic liquid)s: A new material with enhanced and fast CO2 absorption. Chem. Commun. 2005, 26, 3325–3327. [Google Scholar] [CrossRef]
- Majewski, P.W.; Gopinadhan, M.; Osuji, C.O. The Effects of Magnetic Field Alignment on Lithium Ion Transport in a Polymer Electrolyte Membrane with Lamellar Morphology. Polymers 2019, 11, 887. [Google Scholar] [CrossRef] [Green Version]
- Hyun, K.; Höfl, S.; Kahle, S.; Wilhelm, M. Polymer motion as detected via dielectric spectra of 1,4-cis-polyisoprene under large amplitude oscillatory shear (LAOS). J. Non-Newton. Fluid Mech. 2009, 160, 93–103. [Google Scholar] [CrossRef]
- Kuchel, P.W.; Pagès, G.; Nagashima, K.; Velan, S.; Vijayaragavan, V.; Nagarajan, V.; Chuang, K.H. Stejskal-tanner equation derived in full. Concepts Magn. Reson. Part A 2012, 40A, 205–214. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Ammann, C.; Meier, P.; Merbach, A.E. A Simple Multinuclear NMR Thermometer. J. Magn. Reson. 1982, 46, 319–321. [Google Scholar] [CrossRef]
- Sangoro, J.R.; Serghei, A.; Naumov, S.; Galvosas, P.; Kärger, J.; Wespe, C.; Bordusa, F.; Kremer, F. Charge transport and mass transport in imidazolium-based ionic liquids. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008, 77, 51202. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.; Wolf, M.; Gulich, R.; Krohns, S.; Kastner, S.; Lunkenheimer, P.; Loidl, A. Electrode polarization effects in broadband dielectric spectroscopy. Eur. Phys. J. B 2011, 83, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Choi, U.H.; Ye, Y.; La Salas de Cruz, D.; Liu, W.; Winey, K.I.; Elabd, Y.A.; Runt, J.; Colby, R.H. Dielectric and Viscoelastic Responses of Imidazolium-Based Ionomers with Different Counterions and Side Chain Lengths. Macromolecules 2014, 47, 777–790. [Google Scholar] [CrossRef]
- Broadband Dielectric Spectroscopy; Kremer, F.; Schönhals, A. (Eds.) Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540434070. [Google Scholar]
- Krause, C.; Sangoro, J.R.; Iacob, C.; Kremer, F. Charge transport and dipolar relaxations in imidazolium-based ionic liquids. J. Phys. Chem. B 2010, 114, 382–386. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; Buss, H.G.; McCloskey, B.D. The Compensation Effect in the Vogel–Tammann–Fulcher (VTF) Equation for Polymer-Based Electrolytes. J. Phys. Condens. Matter 2017, 50, 3831–3840. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4U, 226–248. [Google Scholar] [CrossRef] [Green Version]
- Kuray, P.; Noda, T.; Matsumoto, A.; Iacob, C.; Inoue, T.; Hickner, M.A.; Runt, J. Ion Transport in Pendant and Backbone Polymerized Ionic Liquids. Macromolecules 2019, 52, 6438–6448. [Google Scholar] [CrossRef]
- Nakamura, K.; Fukao, K.; Inoue, T. Dielectric Relaxation and Viscoelastic Behavior of Polymerized Ionic Liquids with Various Counteranions. Macromolecules 2012, 45, 3850–3858. [Google Scholar] [CrossRef]
- Dyre, J.C. A Simple Model of ac Hopping Conductivity in Disordered Solids. Phys. Lett. 1985, 1985, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Sangoro, J.R.; Iacob, C.; Naumov, S.; Valiullin, R.; Rexhausen, H.; Hunger, J.; Buchner, R.; Strehmel, V.; Kärger, J.; Kremer, F. Diffusion in ionic liquids: The interplay between molecular structure and dynamics. Soft Matter 2011, 7, 1678. [Google Scholar] [CrossRef] [Green Version]
- Sangoro, J.R.; Iacob, C.; Agapov, A.L.; Wang, Y.; Berdzinski, S.; Rexhausen, H.; Strehmel, V.; Friedrich, C.; Sokolov, A.P.; Kremer, F. Decoupling of ionic conductivity from structural dynamics in polymerized ionic liquids. Soft Matter 2014, 10, 3536–3540. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. i. non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Fox, R.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Wheatle, B.K.; Keith, J.R.; Mogurampelly, S.; Lynd, N.A.; Ganesan, V. Influence of Dielectric Constant on Ionic Transport in Polyether-Based Electrolytes. ACS Macro Lett. 2017, 6, 1362–1367. [Google Scholar] [CrossRef]
- Imbrogno, J.; Maruyama, K.; Rivers, F.; Baltzegar, J.R.; Zhang, Z.; Meyer, P.W.; Ganesan, V.; Aoshima, S.; Lynd, N.A. Relationship between Ionic Conductivity, Glass Transition Temperature, and Dielectric Constant in Poly(vinyl ether) Lithium Electrolytes. ACS Macro Lett. 2021, 10, 1002–1007. [Google Scholar] [CrossRef]
- Huang, M.-M.; Jiang, Y.; Sasisanker, P.; Driver, G.W.; Weingärtner, H. Static Relative Dielectric Permittivities of Ionic Liquids at 25 °C. J. Chem. Eng. Data 2011, 56, 1494–1499. [Google Scholar] [CrossRef]
- Wheatle, B.K.; Fuentes, E.F.; Lynd, N.A.; Ganesan, V. Design of Polymer Blend Electrolytes through a Machine Learning Approach. J. Phys. Condens. Matter 2020, 53, 9449–9459. [Google Scholar] [CrossRef]
- Choi, U.H.; Lee, M.; Wang, S.; Liu, W.; Winey, K.I.; Gibson, H.W.; Colby, R.H. Ionic Conduction and Dielectric Response of Poly(imidazolium acrylate) Ionomers. J. Phys. Condens. Matter 2012, 45, 3974–3985. [Google Scholar] [CrossRef]








| Sample Name | PolyIL [wt%] | IL [wt%] | |||
|---|---|---|---|---|---|
| Poly[VBBI]TFSI | 100 | 0 | |||
| 90/10 | 90 | 10 | |||
| 70/30 | 70 | 30 | |||
| 50/50 | 50 | 50 | |||
| 30/70 | 30 | 70 | |||
| 20/80 | 20 | 80 | |||
| 10/90 | 10 | 90 | |||
| [VBBI]TFSI | 0 | 100 |
| Vogel–Fulcher–Tammann | Arrhenius | ||||
|---|---|---|---|---|---|
| Sample | |||||
| Poly[VBBI]TFSI | 43.8 | 22.3 | |||
| 90/10 polyIL/IL | 187.8 | 27.5 | |||
| 70/30 polyIL/IL | 239.5 | 30.3 | |||
| 50/50 polyIL/IL | 282.5 | 31.1 | |||
| 30/70 polyIL/IL | 82.6 | 26.4 | |||
| 20/80 polyIL/IL | 97.3 | 27.3 | |||
| 10/90 polyIL/IL | 91.0 | 26.0 | |||
| [VBBI]TFSI | 70.0 | 24.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, M.; Iacob, C.; Kaysan, G.; Simmler, M.; Nirschl, H.; Guthausen, G.; Wilhelm, M. Charge Transport and Glassy Dynamics in Blends Based on 1-Butyl-3-vinylbenzylimidazolium Bis(trifluoromethanesulfonyl)imide Ionic Liquid and the Corresponding Polymer. Polymers 2022, 14, 2423. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122423
Hoffmann M, Iacob C, Kaysan G, Simmler M, Nirschl H, Guthausen G, Wilhelm M. Charge Transport and Glassy Dynamics in Blends Based on 1-Butyl-3-vinylbenzylimidazolium Bis(trifluoromethanesulfonyl)imide Ionic Liquid and the Corresponding Polymer. Polymers. 2022; 14(12):2423. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122423
Chicago/Turabian StyleHoffmann, Maxi, Ciprian Iacob, Gina Kaysan, Mira Simmler, Hermann Nirschl, Gisela Guthausen, and Manfred Wilhelm. 2022. "Charge Transport and Glassy Dynamics in Blends Based on 1-Butyl-3-vinylbenzylimidazolium Bis(trifluoromethanesulfonyl)imide Ionic Liquid and the Corresponding Polymer" Polymers 14, no. 12: 2423. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14122423