Protective Properties of Copper-Loaded Chitosan Nanoparticles against Soybean Pathogens Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens
Abstract
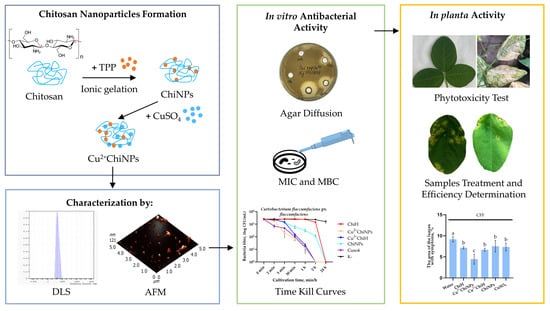
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan Hydrolysate
2.2. Preparation and Characterization of Chitosan Nanoparticles and Copper-Loaded Nanoparticles
2.3. Bacterial Strains
2.4. Determination of Antibacterial Activity of Chitosan Samples
2.4.1. Determination of Antibacterial Activity via Agar Diffusion Method
2.4.2. Determination of Minimum Inhibitory Concentration (MIC)
2.4.3. Determination of Minimum Bactericidal Concentration (MBC)
2.4.4. Determination of Time–Kill Curves
2.5. Phytotoxicity on Soybean Seeds and Plants
2.6. Control Psg and Cff Artificial Infection by Chitosan Samples
2.6.1. Control Psg on Seeds
2.6.2. Control Psg on Leaves
2.6.3. Control Cff on Seeds
2.6.4. Control Cff on Leaves
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation Samples Based on Chitosan
3.2. Antibacterial In Vitro Activity
3.2.1. Determination of Antibacterial In Vitro Activity via Agar Diffusion Method
3.2.2. Determination of Minimum Inhibitory and Bactericidal Concentrations
3.2.3. Antibacterial In Vitro Activity by Determination of Time–Kill Curves
3.3. Phytotoxicity on Seeds and Leaves
3.4. The Efficiency of Chitosan Samples against Psg and Cff Infection on Leaves and Seeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, K.I.; Erh, M.H.; Su, N.W.; Liu, W.H.; Chou, C.C.; Cheng, K.C. Soyfoods and soybean products: From traditional use to modern applications. Appl. Microbiol. Biotechnol. 2012, 96, 9–22. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Roma, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- Hartman, G.L. Diseases of Soybean (Glycine max [L.] Merr.). Available online: https://www.apsnet.org/edcenter/resources/commonnames/Pages/Soybean.aspx (accessed on 23 November 2022).
- Bull, C.T.; De Boer, S.H.; Denny, T.P.; Firrao, G.; Fischer-Le Saux, M.; Saddler, G.S.; Scortichini, M.; Stead, D.E.; Takikawa, Y. Comprehensive list of names of plant pathogenic bacteria, 1980–2007. J. Plant Pathol. 2010, 92, 551–592. [Google Scholar]
- Jagtap, G.P.; Dhopte, S.B.; Dey, U. Bio-efficacy of different antibacterial antibiotic, plant extracts and bioagents against bacterial blight of soybean caused by Pseudomonas syringae pv. glycinea. Sci. J. Microbiol. 2012, 1, 1–9. [Google Scholar]
- Zhang, J.; Wang, X.; Lu, Y.; Bhusal, S.J.; Song, Q.; Cregan, P.B.; Yen, Y.; Brown, M.; Jiang, G.L. Genome-wide Scan for Seed Composition Provides Insights into Soybean Quality Improvement and the Impacts of Domestication and Breeding. Mol. Plant 2018, 11, 460–472. [Google Scholar] [CrossRef] [Green Version]
- Pseudomonas savastanoi pv. glycinea (PSDMGL) [Overview] | EPPO Global Database. Available online: https://gd.eppo.int/taxon/PSDMGL (accessed on 24 November 2022).
- Ignjatov, M.; Milošević, M.; Nikolić, Z.; Vujaković, M.; Petrović, D. Characterization of Pseudomonas savastanoi pv. glycinea isolates From Vojvodina. Phytopathol. Pol. 2007, 45, 43–54. [Google Scholar]
- Shepherd, L.M.; Block, C.C. CHAPTER 13: Detection of Pseudomonas savastanoi pv. glycinea in Soybean Seeds. In Detection of Plant-Pathogenic Bacteria in Seed and Other Planting Material, 2nd ed.; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 85–88. [Google Scholar]
- Huang, H.C.; Erickson, R.S.; Balasubramanian, P.M.; Hsieh, T.F.; Conner, R.L. Resurgence of bacterial wilt of common bean in North America. Can. J. Plant Pathol. 2009, 31, 290–300. [Google Scholar] [CrossRef]
- Soares, R.M.; Fantinato, G.G.P.; Darben, L.M.; Marcelino-Guimarães, F.C.; Seixas, C.D.S.; de Souza Carneiro, G.E. First report of Curtobacterium flaccumfaciens pv. flaccumfaciens on soybean in Brazil. Trop. Plant Pathol. 2013, 38, 452–454. [Google Scholar] [CrossRef]
- Huang, H.C.; Mondel, H.H.; Erickson, R.S.; Chelle, C.D.; Balasubramanian, P.M.; Kiehn, F.; Conner, R.L. Resistance of common bean (Phaseolus vulgaris L.) cultivars and germplasm lines to the purple variant of bacterial wilt (Curtobacterium flaccumfaciens pv. flaccumfaciens). Plant Pathol. Bull. 2007, 16, 91–95. [Google Scholar]
- Camara, R.C.; Vigo, S.C.; Maringoni, A.C. Plant-to-seed transmission of Curtobacterium flaccumfaciens pv. flaccumaciens in a dry bean cultivar. J. Plant Pathol. 2009, 91, 549–554. [Google Scholar] [CrossRef]
- EPPO A2 List. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 28 November 2022).
- Hsieh, T.-F.; Huang, H.C.; Erickson, R.S. Bacterial wilt of common bean: Effect of seedborne inoculum on disease incidence and seedling vigour. Seed Sci. Technol. 2006, 34, 57–67. [Google Scholar] [CrossRef]
- Hsieh, T.-F.; Huang, H.-C.; Mündel, H.-H.; Erickson, R.S. A rapid indoor technique for screening common Bean (Phaseolus vulgaris L.) for resistance to bacterial wilt [Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones]. Rev. Mex. Fitopatol. 2003, 21, 364–369. [Google Scholar]
- Monteil, C.L.; Yahara, K.; Studholme, D.J.; Mageiros, L.; Méric, G.; Swingle, B.; Morris, C.E.; Vinatzer, B.A.; Sheppard, S.K. Population-genomic insights into emergence, crop adaptation and dissemination of Pseudomonas syringae pathogens. Microb. Genom. 2016, 2, e000089. [Google Scholar] [CrossRef] [Green Version]
- Silva Júnior, T.A.F.; Negrão, D.R.; Itako, A.T.; Maringoni, A.C. Pathogenicity of Curtobacterium flaccumfaciens pv. flaccumfaciens to several plant species. J. Plant Pathol. 2012, 94, 427–430. [Google Scholar]
- Urrea, C.A.; Harveson, R.M. Identification of Sources of Bacterial Wilt Resistance in Common Bean (Phaseolus vulgaris). Plant Dis. 2014, 98, 973–976. [Google Scholar] [CrossRef] [Green Version]
- Orzali, L.; Valente, M.T.; Scala, V.; Loreti, S.; Pucci, N. Antibacterial activity of essential oils and trametes versicolor extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for seed treatment and development of a rapid in vivo assay. Antibiotics 2020, 9, 628. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Khan, A.; Ali, N.; Bilal, M.; Malik, S.; Badshah, S.; Iqbal, H.M.N. Engineering Functionalized Chitosan-Based Sorbent Material: Characterization and Sorption of Toxic Elements. Appl. Sci. 2019, 9, 5138. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Khan, A.; Malik, S.; Badshah, S.; Bilal, M.; Iqbal, H.M.N. Chitosan-based green sorbent material for cations removal from an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104064. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2019, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.C.; Boland, A.; Cambier, P.; Frettinger, P.; van Cutsem, P. Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 2010, 20, 775–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hetar, M.Y.; Zainal Abidin, M.A.; Sariah, M.; Wong, M.Y. Antifungal activity of chitosan against Fusarium oxysporum f. sp. cubense. J. Appl. Polym. Sci. 2011, 120, 2434–2439. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.N.; Tung, H.T.; Minh, N.C.; Van Hoa, N.; Phuong, P.T.D.; Trung, T.S. Antibacterial activity of chitosan from squid pens (Loligo chenisis) against Erwinia carotovora from soft rot postharvest tomato fruit. J. Polym. Mater. 2017, 34, 319–330. [Google Scholar]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Preserving apple (Malus domestica var. Anna) fruit bioactive substances using olive wastes extract-chitosan film coating. Inf. Process. Agric. 2017, 4, 90–99. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Francis, J.; Bowrin, V.; Ramjegathesh, R.; Ramsubhag, A.; Jayaraman, J. Bio-efficacy of a chitosan based elicitor on Alternaria solani and Xanthomonas vesicatoria infections in tomato under tropical conditions. Ann. Appl. Biol. 2016, 169, 274–283. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2017, 43, 3527–3537. [Google Scholar] [CrossRef]
- Chidanandappa; Nargund, V.B. Green synthesis of chitosan based copper nanoparticles and their bio-efficacy against bacterial blight of pomegranate. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1298–1305. [Google Scholar] [CrossRef]
- Tarakanov, R.; Shagdarova, B.; Varlamov, V.; Dzhalilov, F. Biocidal and resistance-inducing effects of chitosan on phytopathogens. In Proceedings of the E3S Web of Conferences, Orel, Russian, 24–25 February 2021; Knyazev, S., Loretts, O., Kukhar, V., Panfilova, O., Tsoy, M., Eds.; EDP Sciences: Les Ulis, France, 2021; Volume 254, p. 05007. [Google Scholar]
- Swati; Choudhary, M.K.; Joshi, A.; Saharan, V. Assessment of Cu-Chitosan Nanoparticles for its Antibacterial Activity against Pseudomonas syringae pv. glycinea. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1335–1350. [Google Scholar] [CrossRef]
- Shagdarova, B.T.; Ilyina, A.V.; Lopatin, S.A.; Kartashov, M.I.; Arslanova, L.R.; Dzhavakhiya, V.G.; Varlamov, V.P. Study of the Protective Activity of Chitosan Hydrolyzate Against Septoria Leaf Blotch of Wheat and Brown Spot of Tobacco. Appl. Biochem. Microbiol. 2018, 54, 71–75. [Google Scholar] [CrossRef]
- Lopatin, S.A.; Derbeneva, M.S.; Kulikov, S.N.; Varlamov, V.P.; Shpigun, O.A. Fractionation of chitosan by ultrafiltration. J. Anal. Chem. 2009, 64, 648–651. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Tarakanov, R.I.; Lukianova, A.A.; Pilik, R.I.; Evseev, P.V.; Miroshnikov, K.A.; Dzhalilov, F.S.-U.; Tesic, S.; Ignatov, A. First report of Curtobacterium flaccumfaciens pv. flaccumfaciens causing a bacterial tan spot of soybean in Russia. Plant Dis. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Tarakanov, R.I.; Lukianova, A.A.; Evseev, P.V.; Toshchakov, S.V.; Kulikov, E.E.; Ignatov, A.N.; Miroshnikov, K.A.; Dzhalilov, F.S.-U. Bacteriophage Control of Pseudomonas savastanoi pv. glycinea in Soybean. Plants 2022, 11, 938. [Google Scholar] [CrossRef]
- Sarkar, S.F.; Guttman, D.S. Evolution of the Core Genome of Pseudomonas syringae, a Highly Clonal, Endemic Plant Pathogen. Appl. Environ. Microbiol. 2004, 70, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Evseev, P.; Lukianova, A.; Tarakanov, R.; Tokmakova, A.; Shneider, M.; Ignatov, A.; Miroshnikov, K. Curtobacterium spp. and Curtobacterium flaccumfaciens: Phylogeny, genomics-based taxonomy, pathogenicity, and diagnostics. Curr. Issues Mol. Biol. 2022, 44, 889–927. [Google Scholar] [CrossRef]
- Tegli, S.; Sereni, A.; Surico, G. PCR-based assay for the detection of Curtobacterium flaccumfaciens pv. flaccumfaciens in bean seeds. Lett. Appl. Microbiol. 2002, 35, 331–337. [Google Scholar] [CrossRef]
- Islam, M.; Masum, S.; Rayhan, K.; Haque, Z. Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J. Advaced Sci. Res. 2011, 2, 63–66. [Google Scholar]
- Sowjanya, P.; Srinivasa, B.P.; Lakshmi, N.M. Phytochemical analysis and antibacterial efficacy of Amaranthus tricolor (L.) methanolic leaf extract against clinical isolates of urinary tract pathogens. Afr. J. Microbiol. Res. 2015, 9, 1381–1385. [Google Scholar] [CrossRef] [Green Version]
- CLSI, C.L.S.I. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. CLSI document M07-A9. Clin. Lab. Standars Inst. 2015, 32, 18. [Google Scholar]
- Foerster, S.; Unemo, M.; Hathaway, L.J.; Low, N.; Althaus, C.L. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- ISTA International Rules of Seed. Testing (Supplement rules). Seed Sci. Technol. 1999, 27, 178. [Google Scholar]
- Nalini, S.; Parthasarathi, R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Ann. Agrar. Sci. 2018, 16, 108–115. [Google Scholar] [CrossRef]
- Shine, M.; Fu, D.-Q.; Kachroo, A. Airbrush infiltration method for Pseudomonas syringae Infection Assays in Soybean. Bio-Protocol 2015, 5, e1427. [Google Scholar] [CrossRef]
- Sibiya, M.; Sumbwanyambe, M. An algorithm for severity estimation of plant leaf diseases by the use of colour threshold image segmentation and fuzzy logic inference: A proposed algorithm to update a “Leaf Doctor” application. AgriEngineering 2019, 1, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Tarakanov, R.I.; Lukianova, A.A.; Evseev, P.V.; Pilik, R.I.; Tokmakova, A.D.; Kulikov, E.E.; Toshchakov, S.V.; Ignatov, A.N.; Dzhalilov, F.S.-U.; Miroshnikov, K.A. Ayka, a Novel Curtobacterium Bacteriophage, Provides Protection against Soybean Bacterial Wilt and Tan Spot. Int. J. Mol. Sci. 2022, 23, 913. [Google Scholar] [CrossRef]
- Madden, L.V.; Hughes, G.; van den Bosch, F. The Study of Plant Disease Epidemics; The American Phytopathological Society: Saint Paul, MN, USA, 2017; ISBN 978-0-89054-505-8. [Google Scholar]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Tan, W.N.; Hasanolbasori, M.A.; Abdullah, S.Z. Potential Antimicrobial Applications of Chitosan Nanoparticles (ChNP). J. Microbiol. Biotechnol. 2019, 29, 1009–1013. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- OH, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Jiang, H.; Ye, H.; Li, J.; Huang, J. Preparation, antibacterial, and antioxidant activities of silver/chitosan composites. J. Carbohydr. Chem. 2014, 33, 298–312. [Google Scholar] [CrossRef]
- Qian, J.; Pan, C.; Liang, C. Antimicrobial activity of Fe-loaded chitosan nanoparticles. Eng. Life Sci. 2017, 17, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Katas, H.; Lim, C.S.; Nor Azlan, A.Y.H.; Buang, F.; Mh Busra, M.F. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef]
- Esyanti, R.R.; Farah, N.; Bajra, B.D.; Nofitasari, D.; Martien, R.; Sunardi, S.; Safitri, R. Comparative study of nano-chitosan and synthetic bactericide application on chili pepper (Capsicum annuum L.) infected by xanthomonas campestris. Agrivita 2020, 42, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Vold, I.M.N.; Vårum, K.M.; Guibal, E.; Smidsrød, O. Binding of ions to chitosan—Selectivity studies. Carbohydr. Polym. 2003, 54, 471–477. [Google Scholar] [CrossRef]
- Mekahlia, S.; Bouzid, B. Chitosan-Copper (II) complex as antibacterial agent: Synthesis, characterization and coordinating bond- activity correlation study. Phys. Procedia 2009, 2, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Konovalova, M.; Shagdarova, B.; Zubov, V.; Svirshchevskaya, E. Express analysis of chitosan and its derivatives by gel electrophoresis. Prog. Chem. Appl. Chitin Its Deriv. 2019, 24, 84–95. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Chen, J.; Mao, S.; Xu, Z.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019, 9, 3788–3799. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Eaton, P.; Gomes, A.M.; Pintado, M.E.; Xavier Malcata, F. Study of the antibacterial effects of chitosans on Bacillus cereus (and its spores) by atomic force microscopy imaging and nanoindentation. Ultramicroscopy 2009, 109, 854–860. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Dash, S.; Kumar, M.; Pareek, N. Enhanced antibacterial potential of berberine via synergism with chitosan nanoparticles. Mater. Today Proc. 2019, 31, 640–645. [Google Scholar] [CrossRef]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V.; Subramanian, N.S. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Young, M.; Santra, S. Copper (Cu)–Silica Nanocomposite Containing Valence-Engineered Cu: A New Strategy for Improving the Antimicrobial Efficacy of Cu Biocides. J. Agric. Food Chem. 2014, 62, 6043–6052. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of Copper-Chitosan Nanoparticles Stimulate Growth and Induce Resistance in Finger Millet (Eleusine coracana Gaertn.) Plants against Blast Disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef]
- Shende, S.; Rathod, D.; Gade, A.; Rai, M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotechnol. 2017, 11, 773. [Google Scholar] [CrossRef]
- Swati; Joshi, A. Cu-Chitosan Nanoparticle Induced Plant Growth and Disease Resistance Efficiency of Soybean [Glycine max (L.)]. Legum. Res. 2022, 1, 6. [Google Scholar] [CrossRef]
- Kumar, N.V.; Basavegowda, V.R.; Murthy, A.N.; Lokesh, S. Synthesis and characterization of copper-chitosan based nanofungicide and its induced defense responses in Fusarium wilt of banana. Inorg. Nano-Met. Chem. 2022, 1–9. [Google Scholar] [CrossRef]





| Samples | Relative Concentrations of Samples, % (v/v) | Concentration of Chitosan, mg/mL | Concentration of CuSO4, mg/mL |
|---|---|---|---|
| ChiH | 100 | 5 | - |
| 75 | 3.75 | - | |
| 50 | 2.5 | - | |
| 25 | 1.25 | - | |
| 10 | 0.5 | - | |
| 1 | 0.05 | - | |
| Cu2+ChiH | 100 | 5 | 0.83 |
| 75 | 3.75 | 0.62 | |
| 50 | 2.5 | 0.42 | |
| 25 | 1.25 | 0.21 | |
| 10 | 0.5 | 0.083 | |
| 1 | 0.05 | 0.0083 | |
| ChiNPs | 100 | 5 | - |
| 75 | 3.75 | - | |
| 50 | 2.5 | - | |
| 25 | 1.25 | - | |
| 10 | 0.5 | - | |
| 1 | 0.05 | - | |
| Cu2+ChiNPs | 100 | 5 | 0.83 |
| 75 | 3.75 | 0.62 | |
| 50 | 2.5 | 0.42 | |
| 25 | 1.25 | 0.21 | |
| 10 | 0.5 | 0.083 | |
| 1 | 0.05 | 0.0083 | |
| CuSO4 | 100 | - | 0.83 |
| 75 | - | 0.62 | |
| 50 | - | 0.42 | |
| 25 | - | 0.21 | |
| 10 | - | 0.083 | |
| 1 | - | 0.0083 |
| Samples | Size, nm | Polydispersity Index | Zeta-Potential, mV |
|---|---|---|---|
| ChiNPs | 254 ± 37 | 0.499 | 37.8 ± 1.6 |
| Cu2+ChiNPs | 153 ± 30 | 0.421 | 22.7 ± 0.4 |
| ChiNPs cf * | 251 ± 32 | 0.367 | 48.5 ± 0.6 |
| Cu2+ChiNPs cf * | 157 ± 42 | 0.540 | 27.2 ± 0.6 |
| Samples | Minimal Inhibitory (MIC) and Bactericidal (MBC) Concentrations of Samples, µg/mL (Chitosan/Copper) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Psg Strains | Cff Strains | |||||||||||
| CFBP 2214 | G2 | G17 | CFBP 2214 | G2 | G17 | CFBP 3418 | F-125-1 | F-30-1 | CFBP 3418 | F-125-1 | F-30-1 | |
| MIC | MBC | MIC | MBC | |||||||||
| ChiH | 156/- | 156/- | 156/- | 625/- | 625/- | 625/- | 78/- | 78/- | 78/- | 312/- | 312/- | 312/- |
| Cu2+ChiH | 78/13 | 78/13 | 78/13 | 78/13 | 78/13 | 39/6 | 19/3 | 19/3 | 19/3 | 312/52 | 312/52 | 0.321/52 |
| ChiNPs | 39/- | 39/- | 39/- | 156/- | 156/- | 156/- | 39/- | 39/- | 39/- | 156/- | 156/- | 156/- |
| Cu2+ChiNPs | 19/3 | 19/3 | 19/3 | 78/13 | 78/13 | 78/13 | 19/3 | 19/3 | 19/3 | 78/13 | 78/13 | 78/13 |
| CuSO4 | -/6 | -/13 | -/3 | -/13 | -/26 | -/13 | -/13 | -/13 | -/13 | -/52 | -/52 | -/52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarakanov, R.; Shagdarova, B.; Lyalina, T.; Zhuikova, Y.; Il’ina, A.; Dzhalilov, F.; Varlamov, V. Protective Properties of Copper-Loaded Chitosan Nanoparticles against Soybean Pathogens Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens. Polymers 2023, 15, 1100. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051100
Tarakanov R, Shagdarova B, Lyalina T, Zhuikova Y, Il’ina A, Dzhalilov F, Varlamov V. Protective Properties of Copper-Loaded Chitosan Nanoparticles against Soybean Pathogens Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens. Polymers. 2023; 15(5):1100. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051100
Chicago/Turabian StyleTarakanov, Rashit, Balzhima Shagdarova, Tatiana Lyalina, Yuliya Zhuikova, Alla Il’ina, Fevzi Dzhalilov, and Valery Varlamov. 2023. "Protective Properties of Copper-Loaded Chitosan Nanoparticles against Soybean Pathogens Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens" Polymers 15, no. 5: 1100. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051100