Fabrication of Fibrin/Polyvinyl Alcohol Scaffolds for Skin Tissue Engineering via Emulsion Templating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibrin/PVA Scaffold Manufacture
2.2. Fourier Transform-Infrared Spectroscopy (FT-IR) Analysis
2.3. Microstructure and Pore Size of Fibrin/PVA Scaffold
2.4. Mechanical Properties of Fibrin/PVA Scaffold
2.5. Degradation Profile of Fibrin/PVA Scaffold
2.6. Cell Experiments
2.6.1. Cell Culture
2.6.2. Cell Proliferation in Fibrin/PVA Scaffolds
2.6.3. Cell Morphology on Fibrin/PVA Scaffolds
2.7. Establishment of Full-Thickness Skin Wound Model and Would Healing Evaluation
2.8. Histological Staining Analysis
2.9. Immunofluorescence Staining
2.10. Statistics
3. Results and Discussion
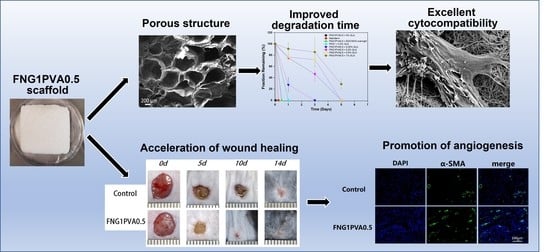
3.1. Scaffold Composition, Structure, and Porosity
3.2. Mechanical Properties of Scaffold
3.3. Enzymatic Degradation of Scaffold
3.4. Cytocompatibility of Scaffolds
3.5. The In Vivo Wound Healing Efficacy of the Fibrin/PVA Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. WIREs Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier function of the skin:“La raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, J.A.; Graetz, N.; Bolliger, I.; Naghavi, M.; Higashi, H.; Mullany, E.C.; Abera, S.F.; Abraham, J.P.; Adofo, K.; Alsharif, U.; et al. The global burden of injury: Incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj. Prev. 2016, 22, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Ranjkesh, M.R.; Khosroshahi, A.F. Skin Burns: Review of Molecular Mechanisms and Therapeutic Approaches. Wounds A Compend. Clin. Res. Pract. 2019, 31, 308–315. [Google Scholar]
- Halim, A.S.; Khoo, T.; Yussof, S.J.M. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. Off. Publ. Assoc. Plast. Surg. India 2010, 43, S23. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [Green Version]
- Frykberg, R.G.; Banks, J.; Deptuła, M.; Karpowicz, P.; Wardowska, A.; Sass, P.; Sosnowski, P.; Mieczkowska, A.; Filipowicz, N.; Dzierżyńska, M.; et al. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R.; Chaudhari, A.A.; Vig, K.; et al. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Ruiz-Velasco, G.; Martínez-Flores, F.; Morales-Corona, J.; Olayo-Valles, R.; Olayo, R. Polymeric Scaffolds For Skin. Macromolecular Symposia 2017, 374, 1600133. [Google Scholar] [CrossRef]
- Liu, H.; Mao, J.; Yao, K.; Yang, G.; Cui, L.; Cao, Y. A study on a chitosan-gelatin-hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 25–40. [Google Scholar] [CrossRef]
- Shalumon, K.; Anulekha, K.; Chennazhi, K.; Tamura, H.; Nair, S.; Jayakumar, R.; Shalumon, K.; Anulekha, K.; Chennazhi, K.; Tamura, H.; et al. Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 571–576. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Sharma, K.; Bullock, A.J.; Giblin, V.; MacNeil, S. Identification of a fibrin concentration that promotes skin cell outgrowth from skin explants onto a synthetic dermal substitute. JPRAS Open 2020, 25, 8–17. [Google Scholar] [CrossRef]
- Han, C.-M.; Zhang, L.-P.; Sun, J.-Z.; Shi, H.-F.; Zhou, J.; Gao, C.-Y. Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. J. Zhejiang Univ. B 2010, 11, 524–530. [Google Scholar] [CrossRef]
- Ahmed, T.A.; EDare, V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Dye, J.F. From Secondary Intent to Accelerated Regenerative Healing: Emergence of the Bio-intelligent Scaffold Vasculogenic Strategy for Skin Reconstruction, in Vascularization for Tissue Engineering and Regenerative Medicine; Holnthoner, W., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–68. [Google Scholar]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2013, 10, 1502–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpalo, E.; Bidault, L.; Boissière, M.; Vancaeyzeele, C.; Fichet, O.; Garde, V.L. Fibrin–polyethylene oxide interpenetrating polymer networks: New self-supported biomaterials combining the properties of both protein gel and synthetic polymer. Acta Biomater. 2011, 7, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, M.; Peters, G.; Rutten, M.; Baaijens, F. A knitted, fibrin-covered polycaprolactone scaffold for tissue engineering of the aortic valve. Tissue Eng. 2006, 12, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Mirhaj, M.; Labbaf, S.; Varshosaz, J.; Taymori, S.; Jafarpour, F.; Salehi, S.; Abadi, S.A.M.; Sepyani, A. Fabrication and evaluation of Cs/PVP sponge containing platelet-rich fibrin as a wound healing accelerator: An in vitro and in vivo study. Int. J. Biol. Macromol. 2022, 204, 245–257. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhao, J.-Y.; Hu, W.-Z.; Ma, K.; Chao, Y.; Sun, P.-J.; Fu, X.-B.; Zhang, H. Synthetic poly(vinyl alcohol)–chitosan as a new type of highly efficient hemostatic sponge with blood-triggered swelling and high biocompatibility. J. Mater. Chem. B 2019, 7, 1855–1866. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Duboeuf, F.; Basarab, A.; Liebgott, H.; Brusseau, E.; Delachartre, P.; Vray, D. Investigation of PVA cryogel Young’s modulus stability with time, controlled by a simple reliable technique. Med. Phys. 2009, 36, 656–661. [Google Scholar] [CrossRef]
- Choi, S.M.; Singh, D.; Kumar, A.; Oh, T.H.; Cho, Y.W.; Han, S.S. Porous Three-Dimensional PVA/Gelatin Sponge for Skin Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 384–389. [Google Scholar] [CrossRef]
- Bidault, L.; Deneufchatel, M.; Vancaeyzeele, C.; Fichet, O.; Larreta-Garde, V. Self-Supported Fibrin-Polyvinyl Alcohol Interpenetrating Polymer Networks: An Easily Handled and Rehydratable Biomaterial. Biomacromolecules 2013, 14, 3870–3879. [Google Scholar] [CrossRef]
- Talukder, M.E.; Hasan, K.M.F.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Li, Z.; Tang, P.; Tang, Y.; Zhang, Y.; Nie, X.; Fang, C.; Li, X.; Zhang, H. Konjac glucomannan/polyvinyl alcohol nanofibers with enhanced skin healing properties by improving fibrinogen adsorption. Mater. Sci. Eng. C 2020, 110, 110718. [Google Scholar] [CrossRef]
- Xu, F.; Zou, D.; Dai, T.; Xu, H.; An, R.; Liu, Y.; Liu, B. Effects of incorporation of granule-lyophilised platelet-rich fibrin into polyvinyl alcohol hydrogel on wound healing. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Richez, A.; Vedrenne, P.; Collier, R. Preparation of ultra-low-density microcellular materials. J. Appl. Polym. Sci. 2005, 96, 2053–2063. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Aslam, H.Z.; Ansari, T.M.; Zhang, H.; Hussain, I. Fundamentals and Design-Led Synthesis of Emulsion-Templated Porous Materials for Environmental Applications. Adv. Sci. 2021, 8, 2102540. [Google Scholar] [CrossRef]
- Dikici, B.A.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef]
- Lim, X.; Potter, M.; Cui, Z.; Dye, J.F. Manufacture and characterisation of EmDerm-novel hierarchically structured bio-active scaffolds for tissue regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 79. [Google Scholar] [CrossRef] [Green Version]
- Caetano, G.; Violante, R.; Sant´ana, A.B.; Murashima, A.B.; Domingos, M.; Gibson, A.; Bártolo, P.; Frade, M.A. Cellularized versus decellularized scaffolds for bone regeneration. Mater. Lett. 2016, 182, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Abureesh, M.A.; Oladipo, A.A.; Gazi, M. Facile synthesis of glucose-sensitive chitosan–poly(vinyl alcohol) hydrogel: Drug release optimization and swelling properties. Int. J. Biol. Macromol. 2016, 90, 75–80. [Google Scholar] [CrossRef]
- Su, L.; Xing, Z.; Wang, D.; Xu, G.; Ren, S.; Fang, G. Mechanical Properties Research and Structural Characterization of Alkali Lignin / Poly(vinyl alcohol) Reaction Films. Bioresources 2013, 8, 3532–3543. [Google Scholar] [CrossRef]
- Luo, Q.; Shan, Y.; Zuo, X.; Liu, J. Anisotropic tough poly(vinyl alcohol)/graphene oxide nanocomposite hydrogels for potential biomedical applications. RSC Adv. 2018, 8, 13284–13291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbag, I.; Saleh, S.M. Studies on the formation of intermolecular interactions and structural characterization of polyvinyl alcohol/lignin film. Int. J. Environ. Stud. 2016, 73, 1–10. [Google Scholar] [CrossRef]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and precipitation in polysaccharide–protein systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 492–502. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R. Fibrin Formation, Structure and Properties, in Fibrous Proteins: Structures and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2017; pp. 405–456. [Google Scholar]
- Yang, J.; Wang, F.; Tan, T. Controlling degradation and physical properties of chemical sand fixing agent-poly(aspartic acid) by crosslinking density and composites. J. Appl. Polym. Sci. 2008, 111, 1557–1563. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Zhu, J.; Inverarity, C.; Fang, Y.; Zhang, Z.; Ye, H.; Cui, Z.; Nguyen, L.; Wan, H.; Dye, J.F. Fabrication of Fibrin/Polyvinyl Alcohol Scaffolds for Skin Tissue Engineering via Emulsion Templating. Polymers 2023, 15, 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051151
Zhou G, Zhu J, Inverarity C, Fang Y, Zhang Z, Ye H, Cui Z, Nguyen L, Wan H, Dye JF. Fabrication of Fibrin/Polyvinyl Alcohol Scaffolds for Skin Tissue Engineering via Emulsion Templating. Polymers. 2023; 15(5):1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051151
Chicago/Turabian StyleZhou, Guoying, Jiayan Zhu, Catriona Inverarity, Yifeng Fang, Zhao Zhang, Hua Ye, Zhanfeng Cui, Linh Nguyen, Haitong Wan, and Julian F. Dye. 2023. "Fabrication of Fibrin/Polyvinyl Alcohol Scaffolds for Skin Tissue Engineering via Emulsion Templating" Polymers 15, no. 5: 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051151