A Fluorinated Polyimide Based Nano Silver Paste with High Thermal Resistance and Outstanding Thixotropic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fluorinated Polyamide Acid (FPAA) Resin
2.3. Preparation of Nano Silver Pastes
2.4. Measurements
3. Results and Discussion
3.1. Dispersion Homogeneity
3.2. Electrical Conductivity
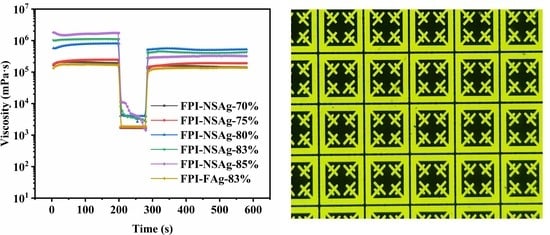
3.3. Rheological Properties
3.4. Thermal Endurance
3.5. Mechanical Properties of Printing Pattern
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beniwal, A.; Ganguly, P.; Aliyana, A.K.; Khandelwal, G.; Dahiya, R. Screen-printed graphene-carbon ink based disposable humidity sensor with wireless communication. Sens. Actuators B Chem. 2023, 374, 132731. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, L.; Qian, J.; Peng, J.; Liu, R.; Xu, Y.; Shi, X.; Qi, C.; Ye, S. Tuned transport behavior of the IPA-treated PEDOT:PSS flexible temperature sensor via screen printing. J. Electron. Mater. 2021, 50, 2356–2364. [Google Scholar] [CrossRef]
- Zhou, H.; Qin, W.; Yu, Q.; Cheng, H.; Yu, X.; Wu, H. Transfer printing and its applications in flexible electronic devices. Nanomaterials 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, H.; Kim, J.H.; Yeo, W.H. Advanced nanomaterials, printing processes, and applications for flexible hybrid electronics. Materials 2020, 13, 3587. [Google Scholar] [CrossRef]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly conductive and transparent PEDOT:PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Shin, K.Y.; Lee, J.S.; Hong, J.Y.; Jang, J. One-step fabrication of a highly conductive and durable copper paste and its flexible dipole tag-antenna application. Chem. Commun. 2014, 50, 3093–3096. [Google Scholar] [CrossRef]
- Morag, A.; Ezersky, V.; Froumin, N.; Mogiliansky, D.; Jelinek, R. Transparent, conductive gold nanowire networks assembled from soluble Au thiocyanate. Chem. Commun. 2013, 49, 8552–8554. [Google Scholar] [CrossRef]
- Im, J.; Trindade, G.F.; Quach, T.T.; Sohaib, A.; Wang, F.; Austin, J.; Turyanska, L.; Roberts, C.J.; Wildman, R.; Hague, R.; et al. Functionalized Gold Nanoparticles with a Cohesion Enhancer for Robust Flexible Electrodes. ACS Appl. Nano Mater. 2022, 5, 6708–6716. [Google Scholar] [CrossRef]
- Alomainy, A. Screen printing carbon nanotubes textiles antennas for smart wearables. Sensors 2021, 21, 4934. [Google Scholar]
- Liu, L.; Shen, Z.; Zhang, X.; Ma, H. Highly conductive graphene/carbon black screen printing inks for flexible electronics. J. Colloid Interface Sci. 2020, 582 Pt A, 12–21. [Google Scholar] [CrossRef]
- Tepner, S.; Wengenmeyr, N.; Linse, M.; Lorenz, A.; Pospischil, M.; Clement, F. The Link between Ag-paste rheology and screen-printed solar cell metallization. Adv. Mater. Technol. 2020, 5, 2000654. [Google Scholar] [CrossRef]
- Walker, S.B.; Lewis, J.A. Reactive silver inks for patterning high-conductivity features at mild temperatures. J. Am. Chem. Soc. 2012, 134, 1419–1421. [Google Scholar] [CrossRef]
- Tang, Y.; He, W.; Wang, S.; Tao, Z.; Cheng, L. One step synthesis of silver nanowires used in preparation of conductive silver paste. J. Mater. Sci. Mater. Electron. 2014, 25, 2929–2933. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, H.E.; Wang, S.; Chen, Y.; Ke, H.U. Preparation of epoxy-silver composite conductive silver paste. Electron. Compon. Mater. 2010, 29, 54–56. [Google Scholar]
- Fang, Z.; Lin, Z.; Peng, Z. Preparation and characterization of low temperature curing conductive silver paste for screen printing. In Proceedings of the 2018 International Symposium On Mechanics, Structures and Materials Science (MSMS 2018), Tianjin, China, 9 June 2018. [Google Scholar]
- Li, H.; Zhu, X.; Li, Z.; Yang, J.; Lan, H. Preparation of nano silver paste and applications in transparent electrodes via electric-field driven micro-scale 3D printing. Nanomaterials 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Xing, J.; Zhang, J.; Xiong, S.; Yang, Y.; Yuan, X.; Li, H.; Tong, H. Microscopic investigation on sintering mechanism of electronic silver paste and its effect on electrical conductivity of sintered electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 18540–18546. [Google Scholar] [CrossRef]
- Lin, Y.; Jean, J. Constrained sintering of silver circuit paste. J. Am. Ceram. Soc. 2010, 87, 187–191. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, Y.; Li, M.; Xu, H.; Li, Y. Synthesis of PVZ glass and its improvement on mechanical and electrical properties of low temperature sintered silver paste. J. Mater. Sci. Mater. Electron. 2020, 31, 8086–8098. [Google Scholar] [CrossRef]
- Liang, J.; Tong, K.; Pei, Q. A water-based silver-nanowire screen-print ink for the fabrication of stretchable conductors and wearable thin-film transistors. Adv. Mater. 2016, 28, 5986–5996. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Qi, X.; Li, H.; Zhang, Y.F.; Li, Z.; Peng, Z.; Yang, J.; Qian, L.; Xu, Q.; et al. Templateless, plating-free fabrication of flexible transparent electrodes with embedded silver mesh by electric-field-driven microscale 3D printing and hybrid hot embossing. Adv. Mater. 2021, 33, 2007772. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Ma, B.; Pei, Y.; Ai, C.; Yuan, L. Fabrication of micron-SiO 2 @nano-Ag based conductive line patterns through silk-screen printing. RSC Adv. 2014, 4, 47781–47787. [Google Scholar] [CrossRef]
- Wu, H.; Chiang, S.W.; Lin, W.; Yang, C.; Li, Z.; Liu, J.; Cui, X.; Kang, F.; Wong, C.P. Towards practical application of paper based printed circuits: Capillarity effectively enhances conductivity of the thermoplastic electrically conductive adhesives. Sci. Rep. 2014, 4, 6275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, R.; Moon, K.S.; Liu, Y.; Hansen, K.; Le, T.; Wong, C.P. Highly conductive, flexible, polyurethane-based adhesives for flexible and printed electronics. Adv. Funct. Mater. 2013, 23, 1459–1465. [Google Scholar] [CrossRef]
- Chen, S.; Liu, K.; Luo, Y.; Wei, Y.; Li, F.; Liu, L. Construction of silver nanochains on DNA template for flexible electrical conductive composites. Mater. Lett. 2015, 147, 109–112. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Cortes, L.Q.; Lonjon, A.; Dantras, E.; Lacabanne, C. High conductive Ag nanowire-polyimide composites: Charge transport mechanism in thermoplastic thermostable materials. J. Non-Cryst. Solids. 2014, 385, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ding, G.; Yang, Z. A high sensitive flexible pressure sensor designed by silver nanowires embedded in polyimide (AgNW-PI). Micromachines 2019, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.L.; Hsu, S.L. Preparation and properties of conductive silver/photosensitive polyimide nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1575–1583. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-time characterization of physical changes in polyimide film formation: From casting to imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, W.; Zhang, B.Q.; Yang, S.Y.; Liu, C.Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, X.; Yang, H.X.; Zhao, J.; Yang, S.Y. The in-plane orientation and thermal mechanical properties of the chemically imidized polyimide films. Chin. J. Polym. Sci. 2019, 37, 11. [Google Scholar] [CrossRef]













| Mass of Each Component (g) | |||||
|---|---|---|---|---|---|
| Notes | Nano Silver | Flake Silver | FPAA Resin a | BYK-333 | BYK-066N |
| FPI-NSAg-70% | 93.3 | 0 | 100 | 0.97 | 0.97 |
| FPI-NSAg-75% | 120 | 0 | 100 | 1.10 | 1.10 |
| FPI-NSAg-80% | 160.0 | 0 | 100 | 1.30 | 1.30 |
| FPI-NSAg-83% | 195.3 | 0 | 100 | 1.48 | 1.48 |
| FPI-NSAg-85% | 226.7 | 0 | 100 | 1.63 | 1.63 |
| FPI-FAg-83% | 0 | 195.3 | 100 | 1.48 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, D.; Zhang, C.; Chen, W.; Meng, Q.; Yuan, H.; Yang, S. A Fluorinated Polyimide Based Nano Silver Paste with High Thermal Resistance and Outstanding Thixotropic Performance. Polymers 2023, 15, 1150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051150
Wang Z, Wang D, Zhang C, Chen W, Meng Q, Yuan H, Yang S. A Fluorinated Polyimide Based Nano Silver Paste with High Thermal Resistance and Outstanding Thixotropic Performance. Polymers. 2023; 15(5):1150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051150
Chicago/Turabian StyleWang, Zhenhe, Dong Wang, Chunbo Zhang, Wei Chen, Qingjie Meng, Hang Yuan, and Shiyong Yang. 2023. "A Fluorinated Polyimide Based Nano Silver Paste with High Thermal Resistance and Outstanding Thixotropic Performance" Polymers 15, no. 5: 1150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15051150