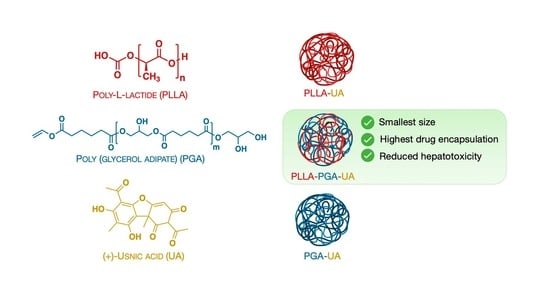
Nanostructured Poly-l-lactide and Polyglycerol Adipate Carriers for the Encapsulation of Usnic Acid: A Promising Approach for Hepatoprotection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nuclear Magnetic Resonance (NMR)
2.2.2. Attenuated Total Reflectance–Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Synthesis of Polyglycerol Adipate
2.2.5. Nanoparticles’ Preparation
2.2.6. Usnic Acid Encapsulation in Polymer Nanoparticles
2.2.7. Dynamic Light Scattering (DLS)
2.2.8. Enzymatic Degradation
2.2.9. Apparent Solubility Test
2.2.10. Study of Drug Release
2.2.11. Cell Culture and MTS Assay
2.2.12. Immunofluorescence Analysis
3. Results and Discussion
3.1. Polyglycerol Adipate Synthesis
3.2. FTIR Spectroscopy and Thermal Properties of Polymers Alone and Blended
3.3. Size and Zeta Potential of PLLA, PGA, and PLLA-PGA Nanoparticles
3.4. Qualitative Degradation Assay of PLLA, PGA, and PLLA-PGA Nanoparticles
3.5. Encapsulation of UA into PLLA, PGA, and PLLA-PGA Nanoparticles
3.6. Thermal Properties of UA-Loaded Nanoparticles
3.7. Drug Release from nanoparticles
3.8. In Vitro Cytotoxicity Test
3.9. Morphological Assessment of HepG2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef] [PubMed]
- Machtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Preman, N.K.; Jain, S.; Johnson, R.P. “Smart” Polymer Nanogels as Pharmaceutical Carriers: A Versatile Platform for Programmed Delivery and Diagnostics. ACS Omega 2021, 6, 5075–5090. [Google Scholar] [CrossRef] [PubMed]
- Sturabotti, E.; Moldoveanu, V.G.; Camilli, A.; Martinelli, A.; Simonetti, G.; Valletta, A.; Serangeli, I.; Giustini, A.; Miranda, E.; Migneco, L.M.; et al. Thymol-Functionalized Hyaluronic Acid as Promising Preservative Biomaterial for the Inhibition of Candida albicans Biofilm Formation. ACS Macro Lett. 2023, 12, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Cautela, J.; Stenqvist, B.; Schillén, K.; Belić, D.; Månsson, L.K.; Hagemans, F.; Seuss, M.; Fery, A.; Crassous, J.J.; Galantini, L. Supracolloidal Atomium. ACS Nano 2020, 14, 15748–15756. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Belić, D.; Del Giudice, A.; Alfredsson, V.; Carnerup, A.M.; Zhu, K.; Nyström, B.; Wang, Y.; Galantini, L.; Schillén, K. Condensed supramolecular helices: The twisted sisters of DNA. Angew. Chem. 2022, 134, e202113279. [Google Scholar] [CrossRef]
- Tasca, E.; Andreozzi, P.; Del Giudice, A.; Galantini, L.; Schillén, K.; Maria Giuliani, A.; Ramirez, M.L.A.; Moya, S.E.; Giustini, M. Poloxamer/sodium cholate co-formulation for micellar encapsulation of doxorubicin with high efficiency for intracellular delivery: An in-vitro bioavailability study. J. Colloid. Interface Sci. 2020, 579, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- Jeong, J.-C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly(ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef]
- Vollrath, A.; Kretzer, C.; Beringer-Siemers, B.; Shkodra, B.; Czaplewska, J.A.; Bandelli, D.; Stumpf, S.; Hoeppener, S.; Weber, C.; Werz, O.; et al. Effect of crystallinity on the properties of polycaprolactone nanoparticles containing the dual FLAP/mPEGS-1 Inhibitor BRP-187. Polymers 2021, 13, 2557. [Google Scholar] [CrossRef]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsdóttir, K. Molecules of interest. Usnic acid. Phytochemistry 2002, 140, 729–736. [Google Scholar] [CrossRef]
- Noël, A.; Garnier, A.; Clément, M.; Rouaud, I.; Sauvager, A.; Bousarghin, L.; Vásquez-Ocmín, P.; Maciuk, A.; Tomasi, S. Lichen-associated bacteria transform antibacterial usnic acid to products of lower antibiotic activity. Phytochemistry 2021, 181, 112535. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nguyen, T.T.; Jeong, M.H.; Crişan, F.; Yu, Y.H.; Ha, H.H.; Choi, K.H.; Jeong, H.G.; Jeong, T.C.; Lee, K.Y.; et al. Inhibitory Activity of (+)-Usnic Acid against Non-Small Cell Lung Cancer Cell Motility. PLoS ONE 2016, 11, e0146575. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.P.; Wang, C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ. Toxicol. Pharmacol. 2020, 80, 103493. [Google Scholar] [CrossRef]
- Favreau, J.T.; Ryu, M.L.; Braunstein, G.; Orshansky, G.; Park, S.S.; Coody, G.L.; Love, L.A.; Fong, T.L. Severe hepatotoxicity associate with the dietary supplement LipoKinetix. Ann. Intern. Med. 2002, 13, 590–595. [Google Scholar] [CrossRef]
- Wang, H.; Xuan, M.; Huang, C.; Wang, C. Advances in Research on Bioactivity, Toxicity, Metabolism, and Pharmacokinetics of Usnic Acid In Vitro and In Vivo. Molecules 2022, 27, 7469. [Google Scholar] [CrossRef]
- Zugic, A.; Tadic, V.; Savic, S. Nano- and Microcarriers as Drug Delivery Systems for Usnic Acid: Review of Literature. Pharmaceutics 2020, 12, 156. [Google Scholar] [CrossRef]
- Da Silva Santos, N.P.; Nascimento, S.C.; Wanderley, M.S.; Pontes-Filho, N.T.; da Silva, J.F.; de Castro, C.M.; Pereira, E.C.; da Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. Eur. J. Pharm. Biopharm. 2006, 64, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.C.; Siqueira-Moura, M.P.; Rolim-Santos, H.M.; Galetti, F.C.; Simioni, A.R.; Santos, N.P.; Tabosa Do Egito, E.S.; Silva, C.L.; Tedesco, A.C.; Santos-Magalhães, N.S. In vitro uptake and antimycobacterial activity of liposomal usnic acid formulation. J. Liposome Res. 2009, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Giansanti, L.; Piozzi, A.; Altieri, B.; Mauceri, A.; Mancini, G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf. B Biointerfaces 2019, 181, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, B.; Mariano, A.; Simonis, B.; Bombelli, C.; Sennato, S.; Piozzi, A.; Taresco, V.; Chauhan, V.M.; Howdle, S.M.; Scotto d’Abusco, A.; et al. Self-assembled chitosan-sodium usnate drug delivery nanosystems: Synthesis, characterization, stability studies, in vitro cytotoxicity and in vivo biocompatibility against 143 B cells. Carbohydr. Polym. Technol. Appl. 2023, 6, 100373. [Google Scholar] [CrossRef]
- Ribeiro-Costa, R.M.; Alves, A.J.; Santos, N.P.; Nascimento, S.C.; Gonçalves, E.C.; Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. In vitro and in vivo properties of usnic acid encapsulated into PLGA-microspheres. J. Microencapsul. 2004, 21, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Olalla, Á.S.; Talavera, V.H.; García, D.L.; Torres, E.G.; García, M.F. Glycerol-based enzymatically synthesized renewable polyesters: Control of molecular weight, degree of branching and functional endgroups. Eur. Polym. J. 2022, 170, 111173. [Google Scholar] [CrossRef]
- Perin, G.B.; Felisberti, M.I. Polyesters Inspired by Glycerides: Enzymatic Polycondensation, Structure, Properties, and Nanoparticle Preparation. Macromolecules 2023, 56, 6968–6977. [Google Scholar] [CrossRef]
- Swainson, S.M.E.; Styliari, I.D.; Taresco, V.; Garnett, M.C. Poly (glycerol adipate) (PGA), an Enzymatically Synthesized Functionalizable Polyester and Versatile Drug Delivery Carrier: A Literature Update. Polymers 2019, 11, 1561. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Šiler, P.; Másilko, J.; Risoluti, R.; Vecchio Ciprioti, S. Synthesis, Structural, Morphological and Thermal Characterization of Five Different Silica-Polyethylene Glycol-Chlorogenic Acid Hybrid Materials. Polymers 2021, 13, 1586. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Vecchio Ciprioti, S. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Angelis, D.D.; Mazzeo, L.; Nardozi, P.; Piemonte, V.; Tuffi, R.; Vecchio Ciprioti, S. Catalytic Pyrolysis of a Residual Plastic Waste Using Zeolites Produced by Coal Fly Ash. Catalysts 2020, 10, 1113. [Google Scholar] [CrossRef]
- Sabet, S.S.; Katbab, A.A. Interfacially compatibilized poly(lactic acid) and poly(lactic acid)/polycaprolactone/organoclaynanocomposites with improved biodegradability and barrier properties: Effects of the compatibilizer structural parameters and feeding route. J. Appl. Polym. Sci. 2009, 11, 1954–1963. [Google Scholar] [CrossRef]
- Jacob, P.L.; Cantu, L.A.R.; Pearce, A.K.; He, Y.; Lentz, J.C.; Moore, J.C.; Machado, F.; Rivers, G.; Apebende, E.; Romero Fernandez, M.; et al. Poly (glycerol adipate)(PGA) backbone modifications with a library of functional diols: Chemical and physical effects. Polymer 2021, 228, 123912. [Google Scholar] [CrossRef]
- Sanna, M.; Sicilia, G.; Alazzo, A.; Singh, N.; Musumeci, F.; Schenone, S.; Spriggs, K.A.; Burley, J.C.; Garnett, M.C.; Taresco, V.; et al. Water Solubility Enhancement of Pyrazolo[3,4-d]pyrimidine Derivatives via Miniaturized Polymer-Drug Microarrays. ACS Med. Chem. Lett. 2018, 9, 193–197. [Google Scholar] [CrossRef]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Modi, S.; Anderson, B.D. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef]
- Štampar, M.; Sedighi Frandsen, H.; Rogowska-Wrzesinska, A.; Wrzesinski, K.; Filipič, M.; Žegura, B. Hepatocellular carcinoma (HepG2/C3A) cell-based 3D model for genotoxicity testing of chemicals. Sci. Total Environ. 2021, 755, 143255. [Google Scholar] [CrossRef]
- Sefried, S.; Häring, H.U.; Weigert, C.; Eckstein, S.S. Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. Open Biol. 2018, 8, 180147. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Kline, B.J.; Beckman, E.J.; Russell, A.J. One-step biocatalytic synthesis of linear polyesters with pendant hydroxyl groups. J. Am. Chem. Soc. 1998, 120, 9475–9480. [Google Scholar] [CrossRef]
- Perin, G.B.; Felisberti, M.I. Enzymatic synthesis of poly (glycerol sebacate): Kinetics, chain growth, and branching behavior. Macromolecules 2020, 53, 7925–7935. [Google Scholar] [CrossRef] [PubMed]
- Rodante, F.; Vecchio, S.; Catalani, G.; Tomassetti, M. Compatibility between active components of a commercial drug. Il Farmaco 2002, 57, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.Y.; Song, M.; Zhao, L.G. Testing, characterization and modelling of mechanical behaviour of poly (lactic-acid) and poly (butylene succinate) blends. Mech. Adv. Mater. Mod. Process. 2016, 2, 7. [Google Scholar] [CrossRef]
- Colombo, C.; Dragoni, L.; Gatti, S.; Pesce, R.M.; Rooney, T.R.; Mavroudakis, E.; Ferrari, R.; Moscatelli, D. Tunable Degradation Behavior of PEGylated Polyester-Based Nanoparticles Obtained Through Emulsion Free Radical Polymerization. Ind. Eng. Chem. Res. 2014, 53, 9128–9135. [Google Scholar] [CrossRef]
- Akagi, T.; Higashi, M.; Kaneko, T.; Kida, T.; Akashi, M. Hydrolytic and enzymatic degradation of nanoparticles based on amphiphilic poly(gamma-glutamic acid)-graft-L-phenylalanine copolymers. Biomacromolecules 2006, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, R.; Qian, F.; Desikan, S.; Hussain, M.; Smith, R.L. Estimation of drug solubility in polymers via differential scanning calorimetry and utilization of the fox equation. Pharm. Dev. Technol. 2009, 14, 19–27. [Google Scholar] [CrossRef]
- Rask, M.B.; Knopp, M.M.; Olesen, N.E.; Holm, R.; Rades, T. Comparison of two DSC-based methods to predict drug-polymer solubility. Int. J. Pharm. 2018, 540, 98–105. [Google Scholar] [CrossRef]
- Chieng, N.; Rades, T.; Aaltonen, J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2011, 55, 618–644. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug Deliv. Rev. 2007, 59, 478–487. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]










| Sample | Tg (°C) | cp (J/g) | Tc (°C) | Hc (J/g) | Tm (°C) | Hm(J/g) | (%) |
|---|---|---|---|---|---|---|---|
| PLLA | 55 | 0.16 | 95 | 9.51 | 166 | 16.61 | 7.6 |
| PGA | −32 | 0.55 | - | - | - | - | - |
| PLLA-PGA | −23.4 (PGA) 47 (PLLA) | 0.25 0.02 | 76 | 7.88 | 162 | 31.72 | 25.6 |
| Sample | Hydrodynamic Diameter (nm) | PDI | ς-Potential (mW) |
|---|---|---|---|
| PLLA | 140.0 ± 2.0 | 0.07 ± 0.01 | −34.0 ± 1.0 |
| PGA | 150.0 ± 1.0 | 0.19 ± 0.02 | −13.0 ± 2.0 |
| PLLA-PGA | 111.0 ± 2.0 | 0.14 ± 0.03 | −24.0 ± 2.0 |
| Sample | Hydrodynamic Diameter (nm) | PDI | ς-Potential (mW) |
|---|---|---|---|
| PLLA-UA20X | 188.0 ± 3.0 | 0.15 ± 0.02 | −25.0 ± 1.0 |
| PGA-UA-20X | 160.0 ± 20.0 | 0.40 ± 0.10 | −21.0 ± 8.0 |
| PLLA-PGA-UA20X | 180.0 ± 20.0 | 0.30 ± 0.10 | −23.0 ± 3.0 |
| PLLA-PGA-UA10X | 210.0 ± 20.0 | 0.29 ± 0.04 | −18.0 ± 3.0 |
| PLLA-PGA-UA40X | 270.0 ± 40.0 | 0.37 ± 0.05 | −19.0 ± 2.0 |
| Sample | Tg (°C) | cp (J/g) | Tc (°C) | Hc (J/g) | Tm (°C) | Hm (J/g) | (%) | Tc (°C) | Hc (J/g) | Tm (°C) | ∆Hm (J/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| POLYMER | DRUG | ||||||||||
| UA | - | - | - | - | - | - | - | - | - | 195 | 111.4 |
| PLLA-UA20X | −53 | 0.17 | 97 | 27,04 | 167 | 33.3 | 6.7 | - | - | 192 | 50.2 |
| PGA-UA20X | −14 | 0.40 | - | - | - | - | - | 70 | 34.5 | 175 | 56.8 |
| PLLA-PGA-UA20X | ND * | ND | 162 | 36.3 | ND | ND | ND | 179 | 21.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brugnoli, B.; Perna, G.; Alfano, S.; Piozzi, A.; Galantini, L.; Axioti, E.; Taresco, V.; Mariano, A.; Scotto d’Abusco, A.; Vecchio Ciprioti, S.; et al. Nanostructured Poly-l-lactide and Polyglycerol Adipate Carriers for the Encapsulation of Usnic Acid: A Promising Approach for Hepatoprotection. Polymers 2024, 16, 427. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030427
Brugnoli B, Perna G, Alfano S, Piozzi A, Galantini L, Axioti E, Taresco V, Mariano A, Scotto d’Abusco A, Vecchio Ciprioti S, et al. Nanostructured Poly-l-lactide and Polyglycerol Adipate Carriers for the Encapsulation of Usnic Acid: A Promising Approach for Hepatoprotection. Polymers. 2024; 16(3):427. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030427
Chicago/Turabian StyleBrugnoli, Benedetta, Greta Perna, Sara Alfano, Antonella Piozzi, Luciano Galantini, Eleni Axioti, Vincenzo Taresco, Alessia Mariano, Anna Scotto d’Abusco, Stefano Vecchio Ciprioti, and et al. 2024. "Nanostructured Poly-l-lactide and Polyglycerol Adipate Carriers for the Encapsulation of Usnic Acid: A Promising Approach for Hepatoprotection" Polymers 16, no. 3: 427. https://0-doi-org.brum.beds.ac.uk/10.3390/polym16030427