Control of Problematic Weeds in Mediterranean Vineyards with the Bioherbicide Pelargonic Acid
Abstract
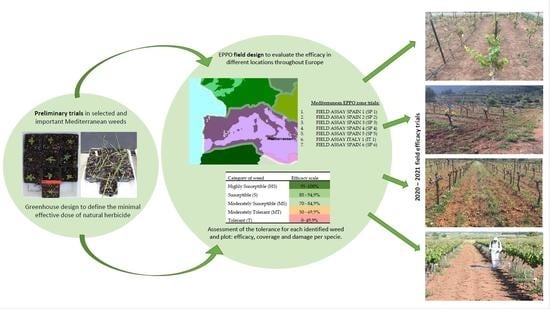
:1. Introduction
2. Materials and Methods
2.1. Post-Emergence Herbicidal Assays against Target Weed Species under Greenhouse Conditions Performed to Obtain Dose–Response Curves
2.1.1. Weeds
2.1.2. Herbicidal Treatments Tested
2.1.3. Evaluation of the Herbicidal Activity
2.2. Post-Emergence Herbicidal Assays against Important Weed Species in Mediterranean Vineyards
2.2.1. Location and Experimental Conditions
2.2.2. Weeds
2.2.3. Treatments
2.2.4. Evaluation of the Herbicidal Activity
- Weed coverage, which is the % of the surface covered by the weeds. This was assessed by visual estimation of the total surface covered by weeds in each treatment (whole area between the rows of 17 vines). This parameter was measured for each trial and pooled by year to compare the stability of the behavior of the formulations.
- Weed density, which is the number of weeds per square meter (plants/m2). The number of individuals from each weed species present in each plot was counted and considered as representative when it was higher than 5 to ensure a minimum number of individuals representative per weed species.
- Weed control or efficacy, which is the percentage of mortality. It was assessed for each individual weed species by visual evaluation of the whole area of treated plots compared with the values found in the untreated plots (UTC). This percentage was measured for each species (both monocots and dicots) and for the total of each plot. The untreated control was settled to compare the weed populations compared with treated plots.
- Photographs of all the experiments were taken as data recording.
2.3. Statistical Analyses
3. Results and Discussion
3.1. Greenhouse Experiment: Herbicidal Efficacy of PA Formulation on A. fatua and C. album Dose–Response Curves
3.2. Field Experiment: Herbicidal Efficacy of a New PA Formulation (PSEI) on Mediterranean Vineyards Weeds
3.2.1. Percentage of Weed Control per Species
3.2.2. Weed Coverage Evolution
Weed Coverage per Trial
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, S.; Pierce, J. Automation: The Future of Weed Control in Cropping Systems; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2014; pp. 249–259. [Google Scholar]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Rust, N.; Lunder, O.E.; Iversen, S.; Vella, S.; Oughton, E.A.; Breland, T.A.; Reed, M.S. Perceived Causes and Solutions to Soil Degradation in the UK and Norway. Land 2022, 11, 131. [Google Scholar] [CrossRef]
- Chen, Y.; Destouni, G.; Goldenberg, R.; Prieto, C. Nutrient source attribution: Quantitative typology distinction of active and legacy source contributions to waterborne loads. Hydrol. Process. 2021, 35, e14284. [Google Scholar] [CrossRef]
- Destouni, G.; Cantoni, J.; Kalantari, Z. Distinguishing active and legacy source contributions to stream water quality: Comparative quantification for chloride and metals. Hydrol. Process. 2021, 35, e14280. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 147, pp. 239–280. [Google Scholar] [CrossRef]
- Dew, D.A. An index of competition for estimating crop loss due to weeds. Can. J. Plant Sci. 1972, 52, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.S.; Orcutt, D.R. Functional relationships of growth and competitiveness in perennial weeds and cotton (Gossypium hirsutum L.). Weed Sci. 1991, 39, 575–584. [Google Scholar] [CrossRef]
- Ekwealor, K.U.; Echereme, C.B.; Ofobeze, T.N.; Okereke, C.N. Economic importance of weeds: A review. Asian J. Plant Sci. 2009, 3, 1–11. [Google Scholar] [CrossRef]
- Kudsk, P.; Streibig, J.C. Herbicides—A two-edged sword. Weed Res. 2003, 43, 90–102. [Google Scholar] [CrossRef]
- Qu, R.-Y.; He, B.; Yang, J.-F.; Lin, H.-Y.; Yang, W.-C.; Wu, Q.-Y.; Li, Q.X.; Yang, G.-F. Where are the new herbicides? Pest Manag. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef]
- Rashid, B.; Husnain, T.; Riazuddin, S. Herbicides and Pesticides as Potential Pollutants: A Global Problem. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 427–447. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. The International Code of Conduct on Pesticide Management, Rome. 2014. Available online: http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/code/en/ (accessed on 3 April 2022).
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable Crop and Weed Management in the Era of the EU Green Deal: A Survival Guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Esposito, M.; Crimaldi, M.; Cirillo, V. Drone and sensor technology for sustainable weed management: A review. Chem. Biol.Technol. Agric. 2021, 8, 18. [Google Scholar] [CrossRef]
- Hartzler, R.; Buhler, D. Ecological management of agricultural weeds. In Ecologically Based Integrated Pest Management; Koul, O., Cuperus, G.W., Eds.; CAB International: Wallinford, UK, 2007; pp. 37–51. [Google Scholar]
- Peeters, A.; Lefebvre, O.; Balogh, L.; Barberi, P.; Batello, C.; Bellon, S.; Giafami, T.; Gkisakis, V.; Lana, M.; Migliorini, P.; et al. A Green Deal for implementing agroecological systems: Reforming the common agricultural policy of the European Union. J. Sustain. Org. Agric. Syst. 2020, 70, 83–93. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.; Singh, S. Use of Natural Products for Weed Management in High-Value Crops: An Overview. Preprints 2018, 2018100737. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Wiley: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance Fatty acids C7 to C18 (approved under Regulation (EC) No 1107/2009 as Fatty acids C7 to C20). EFSA J. 2013, 11, 3023. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Herbicides based on pelargonic acid: Herbicides of the bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1476–1482. [Google Scholar] [CrossRef]
- Coleman, R.; Penner, D. Organic acid enhancement of pelargonic acid. Weed Technol. 2008, 22, 38–41. [Google Scholar] [CrossRef]
- Crmaric, I.; Keller, M.; Krauss, J.; Delabays, N. Efficacy of natural fatty acid based herbicides on mixed weed stands. Jul. Kühn Arch. 2018, 458, 327–332. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez-Moreiras, A.M.; Verdeguer, M. Phytotoxic efects of three natural compounds: Pelargonic acid, carvacrol, and cinnamic aldehyde, against problematic weeds in Mediterranean crops. Agronomy 2020, 10, 791. [Google Scholar] [CrossRef]
- Zorner, P.S.; Tsujino, Y.; Kamioka, O. Novel Herbicidally-Active Esters of Fatty Acids. Canada Patent No. 002134774A, 29 April 1993. [Google Scholar]
- Irzyk, G.P.; Zorner, P.; Kern, A. Scythe Herbicide: A New Contact Herbicide Based Naturally Occurring Pelargonic Acid; WSSA: Englewood, CO, USA, 1997; Volume 37, 103p. [Google Scholar]
- Mohler, C.L. Enhancing the competitive ability of crops. In Ecological Management of Agricultural Weeds; Liebman, M., Mohler, C.L., Staver, C.P., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 269–321. [Google Scholar]
- Kanatas, P.; Travlos, I.; Papastylianou, P.; Gazoulis, I.; Kakabouki, I.; Tsekoura, A. Yield, quality and weed control in soybean crop as affected by several cultural and weed management practices. Not. Bot. Hort Agrobot. 2020, 48, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Verdeguer, M.; Torres-Pagán, N.; Muñoz, M.; Jouini, A.; García-Plasencia, S.; Chinchilla, P.; Berbegal, M.; Salamone, A.; Agnello, S.; Carrubba, A.; et al. Herbicidal Activity of Thymbra capitata (L.) Cav. Essential Oil. Molecules 2020, 25, 2832. [Google Scholar] [CrossRef]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization Standards. Available online: https://www.eppo.int/RESOURCES/eppo_standards (accessed on 10 April 2022).
- Travlos, I.; de Prado, R.; Chachalis, D.; Bilalis, D.J. Herbicide resistance in weeds: Early detection, mechanisms, dispersal, new insights and management issues. Front. Ecol. Evol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Möller, G.; Keasar, T.; Shapira, I.; Möller, D.; Ferrante, M.; Segoli, M. Effect of weed management on the parasitoid community in Mediterranean vineyards. Biology 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Dorado, J.A. trait-based approach in a Mediterranean vineyard: Effects of agricultural management on the functional structure of plant communities. Agric. Ecosyst. Environ. 2021, 316, 107465. [Google Scholar] [CrossRef]
- Lebecque, S.; Lins, L.; Dayan, F.E.; Fauconnier, M.L.; Deleu, M. Interactions between natural herbicides and lipid bilayers mimicking the plant plasma membrane. Front. Plant Sci. 2019, 10, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Renton, M.; Busi, R.; Neve, P.; Thornby, D.; Vila-Aiub, M. Herbicide resistance modelling: Past, present and future. Pest Manag. Sci. 2014, 70, 1394–1404. [Google Scholar] [CrossRef]
- Menne, H.; Köcher, H. HRAC classification of herbicides and resistance development. In Modern Crop Protection Compounds, 2nd ed.; Krämer, W., Schirmer, U., Jeschke, P., Witschel, M., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 5–28. [Google Scholar]
- Tataridas, A.; Jabran, K.; Kanatas, P.; Oliveira, R.S.; Freitas, H.; Travlos, I. Early detection, herbicide resistance screening, and integrated management of Invasive Plant Species: A review. Pest Manag. Sci. 2022, 78, 3957–3972. [Google Scholar] [CrossRef]
- Beckie, H.J.; Ashworth, M.B.; Flower, K.C. Herbicide resistance management: Recent developments and trends. Plants 2019, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org (accessed on 30 August 2022).
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef] [Green Version]
- Knowles, A. Global trends in pesticide formulation technology: The development of safer formulations in China. Outlooks Pest Manag. 2009, 20, 165–170. [Google Scholar] [CrossRef]
- Ebeling, W.; Pence, R.J. Pesticides formulation, influence of formulation on effectiveness. J. Agric. Food Chem. 1953, 1, 386–397. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.H.; Quan, N.V.; Nghia, L.T.; Xuan, T.D. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Xuan, T.D.; Yuichi, O.; Junko, C.; Eiji, T.; Hiroyuki, T.; Mitsuhiro, M.; Khanh, T.D.; Hong, N.H. Kava root (Piper methysticum L.) as a potential natural herbicide and fungicide. J. Crop Prot. 2003, 22, 873–881. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Royo-Esnal, A.; Recasens, J. Herbicidal Effect of Different Alternative Compounds to Control Conyza bonariensis in Vineyards. Agronomy 2022, 12, 960. [Google Scholar] [CrossRef]
- Senseman, S.A. Herbicide Handbook; Weed Science Society of America: Champaign, IL, USA, 2007; pp. 155–157. [Google Scholar]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar] [CrossRef]
- Kanatas, P.; Antonopoulos, N.; Gazoulis, I.; Travlos, I.S. Screening glyphosate-alternative weed control options in important perennial crops. Weed. Sci. 2021, 69, 704–718. [Google Scholar] [CrossRef]
- Barker, A.; Prostak, R. Management of vegetation by alternative practices in fields and roadsides. Int. J. Agron. 2014, 2014, 207828. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.; Mansour, P.; Verdeguer, M. Contact herbicidal activity optimization of methyl capped polyethylene glycol ester of pelargonic acid. J. Plant Dis. Prot. 2022, 1–11. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest. Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]






| Species | Start/End Date | Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Max. | Minim. | Mean | Max. | Minim. | ||
| A. fatua | 10 December 2019– 10 January 2020 | 18.57 | 25.72 | 12.75 | 57.87 | 75.56 | 29.84 |
| C. album | 16 October 2020– 16 November 2020 | 18.90 | 25.52 | 12.30 | 45.88 | 50.26 | 40.40 |
| Treatments | Abbreviations | |
|---|---|---|
| T1 | Control treated with water | CW |
| T2 | 0.7% Pelargonic acid | PSEI 1 |
| T3 | 1.4% Pelargonic acid | PSEI 2 |
| T4 | 2.8% Pelargonic acid | PSEI 3 |
| T5 | 4.5% Pelargonic acid | PSEI 4 |
| T6 | 5.6% Pelargonic acid | PSEI 5 |
| Trial Code | Application Date | Temperature (°C) | Relative Humidity Mean (%) | Precipitation Mean (mm) | |||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | Min. | Max. | Mean | |||
| SP 1 | 1 May 2020 | 15 May 2020 | 13.35 | 24.35 | 18.85 | 76.01 | 0.65 |
| SP 2 | 2 May 2020 | 16 May 2020 | 10.75 | 26.75 | 16.67 | 75.70 | 0.19 |
| SP 3 | 1 June 2020 | 16 June 2020 | 9.51 | 20.38 | 14.55 | 77.30 | 0.78 |
| SP 4 | 3 May 2021 | 17 May 2021 | 11.41 | 25.02 | 18.30 | 65.80 | 1.71 |
| SP 5 | 1 May 2021 | 15 May 2021 | 9.69 | 20.46 | 15.07 | 77.30 | 2.63 |
| IT 1 | 5 May 2021 | 20 May 2021 | 13.25 | 25 | 21.34 | 64.80 | 4.00 |
| SP 6 | 1 September 2021 | 15 September 2021 | 11.41 | 25.02 | 18.22 | 70.50 | 1.71 |
| Guideline | Discipline | Description |
|---|---|---|
| PP 1/135(4) | General standard | Phytotoxicity assessment |
| PP 1/90(3) | Herbicides | Weeds in grapevine |
| PP 1/152(4) | General standard | Design and analysis of efficacy evaluation trials |
| PP 1/181(6) | General standard | Conduct and reporting of efficacy evaluation trials including GEP |
| Treatments | Active Substance (a.s.) | Doses (L/ha) | g of a.s. per ha per Application | Abbreviations |
|---|---|---|---|---|
| T1 | Untreated check | - | - | UTC |
| T2 | 55% Pelargonic acid SEITHOR® | 8 | 4400 | PSEI8 |
| T3 | 55% Pelargonic acid SEITHOR® | 10 | 5500 | PSEI10 |
| T4 | 55%Pelargonic acid SEITHOR® | 12 | 6600 | PSEI12 |
| T5 | 55%Pelargonic acid SEITHOR® | 15 | 8250 | PSEI15 |
| T6 | 68% Pelargonic acid reference Beloukha® | 16 | 10,880 | Ref. 16 |
| Category of Weed | Efficacy Scale |
|---|---|
| Highly Susceptible (HS) | 95–100% |
| Susceptible (S) | 85–94.9% |
| Moderately Susceptible (MS) | 70–84.9% |
| Moderately Tolerant (MT) | 50–69.9% |
| Tolerant (T) | 0–49.9% |
| Trial Code | Identified Weed Species |
|---|---|
| SP 1 | Oxalis pes-caprae L. (OXAPF); Sonchus sp. L. (SOONSS); Cyperus rotundus L. (CYPRO); Bromus sp. L. (BROSS) |
| SP 2 | Chrysantemum fuscatum L. (CHYFU); Chenopodium album L. (CHEAL); Diplotaxis erucoides L. (DIPER) |
| SP 3 | Sonchus arvensis L. (SONAR); Diplotaxis erucoides L. (DIPER); Trifolium sp. L. (1TRFG); Erodium sp. L. (1EROG); Plantago lanceolata L. (PLALA); Hordeum sp. (1HORG) |
| SP 4 | Sonchus oleraceus L. (SONOL); Fumaria officinalis L. (FUMOF); Chrysantemum fuscatum L. (CHYFU) |
| SP 5 | Oxalis pes-caprae L. (OXAPF); Sonchus sp. L. (SONSS); Cyperus rotundus L. (CYPRO); Bromus sp. L. (BROSS) |
| IT 1 | Taraxacum officinale L. (TAROF); Diplotaxis erucoides L. (DIPER); Mercurialis annua L. (MERAN); Convolvulus arvensis L. (CONAR); Lolium perenne L. (LOLPE); Cynodon dactylon L. (CYNDA) |
| SP 6 | Taraxacum officinale L. (TAROF); Diplotaxis erucoides L. (DIPER); Convolvulus arvensis L. (CONAR); Sonchus oleraceus L. (SONOL); Bromus arvensis L. (BROAV) |
| Treatment (L/ha) | g of a.s. per ha per Application | % Control | ||
|---|---|---|---|---|
| Total Monocots | Total Dicots | Total (Monocots and Dicots) | ||
| PSEI 8 | 4400 | 33.67 ± 6.44 c * | 47.92 ± 5.78 c | 40.79 ± 4.68 c |
| PSEI 10 | 5500 | 53.87 ± 7.07 ab | 65.15 ± 4.26 b | 59.51 ± 3.65 b |
| PSEI 12 | 6600 | 58.76 ± 8.27 a | 75.43 ± 3.49 b | 67.09 ± 3.59 b |
| PSEI 15 | 8250 | 70.52 ± 9.52 a | 91.27 ± 1.77 a | 80.90 ± 3.95 a |
| Ref. 16 | 10,880 | 68.88 ± 9.80 a | 87.68 ± 3.10 a | 78.28 ± 3.73 a |
| Average | 57.14 | 73.49 | 65.31 | |
| Dicot Species * | Treatment (L/ha) | ||||
|---|---|---|---|---|---|
| PSEI 8 | PSEI 10 | PSEI 12 | PSEI 15 | Ref. 16 | |
| OXAPF | 45.7 | 77.6 | 78.2 | 85.0 | 83.8 |
| SONSS | 62.6 | 81.9 | 87.5 | 91.3 | 85.0 |
| CHYFU | 72.5 | 77.5 | 92.5 | 87.5 | |
| CHEAL | 82.5 | 88.8 | 100 | 90.0 | |
| DIPER | 65.9 | 77.4 | 85.0 | 99.4 | 90.7 |
| SONAR | 32.0 | 30.8 | 50.0 | 45.0 | |
| 1TRFG | 41.3 | 51.7 | 76.3 | 80.0 | |
| 1EROG | 20.0 | 61.0 | 76.3 | 94.5 | |
| PLALA | 6.3 | 56.3 | 65.8 | 82.5 | |
| 1SONG | 77.5 | 83.8 | 88.8 | 91.3 | 96.3 |
| FUMOF | 53.8 | 62.5 | 62.5 | 81.3 | 93.8 |
| 1CHYG | 61.3 | 73.8 | 73.8 | 87.5 | 93.8 |
| TAROF | 51.3 | 61.9 | 68.8 | 90.7 | 90.6 |
| MERAN | 25.0 | 36.9 | 50.0 | 100 | 100 |
| CONAR | 21.3 | 43.2 | 66.0 | 91.3 | 89.4 |
| SONOL | 77.5 | 85.0 | 95.0 | 100 | |
| Monocot species * | |||||
| CYPRO | 34.4 | 51.9 | 78.2 | 88.8 | 71.3 |
| BROSS | 54.4 | 72.6 | 74.4 | 81.3 | 75.7 |
| 1HORG | 38.3 | 65 | 60 | 100 | |
| LOLPE | 25 | 47.5 | 56.3 | 80 | 83.8 |
| CYNDA | 16.3 | 23.8 | 21.3 | 35 | 33.8 |
| BROAV | 62.5 | 62.5 | 67.5 | 48.8 | |
| Year | Number of Trials | Number of Values | % of Final Coverage (14 DAB) |
|---|---|---|---|
| 2021 | 4 | 19 | 17.28 ± 4.11 a |
| 2020 | 3 | 12 | 19.49 ± 2.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, M.; Torres-Pagán, N.; Jouini, A.; Araniti, F.; Sánchez-Moreiras, A.M.; Verdeguer, M. Control of Problematic Weeds in Mediterranean Vineyards with the Bioherbicide Pelargonic Acid. Agronomy 2022, 12, 2476. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12102476
Muñoz M, Torres-Pagán N, Jouini A, Araniti F, Sánchez-Moreiras AM, Verdeguer M. Control of Problematic Weeds in Mediterranean Vineyards with the Bioherbicide Pelargonic Acid. Agronomy. 2022; 12(10):2476. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12102476
Chicago/Turabian StyleMuñoz, Marta, Natalia Torres-Pagán, Amira Jouini, Fabrizio Araniti, Adela M. Sánchez-Moreiras, and Mercedes Verdeguer. 2022. "Control of Problematic Weeds in Mediterranean Vineyards with the Bioherbicide Pelargonic Acid" Agronomy 12, no. 10: 2476. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy12102476