Genetic Variations Associated with Long-Term Treatment Response in Bipolar Depression
Abstract
:1. Introduction
2. Materials and Methods
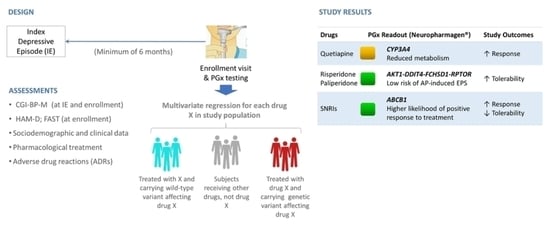
2.1. Study Design
2.2. Subjects
2.3. Data Collection
2.4. Genotyping and Reporting of Results
2.5. Statistics
2.5.1. Predictor Variables
- Gender (male/female);
- Age (years);
- Presence of other psychiatric comorbidities (yes/no);
- Presence of non-psychiatric comorbidities (yes/no);
- Smoking (number of cigarettes per day at enrolment);
- Alcohol consumption (number of standard units per day at enrollment);
- Previous bipolar episodes (number);
- Type of bipolar disorder (I or II);
- Overall CGI-BP score at the onset of the IE;
- Presence of suicidal ideation (yes/no);
- Use of anxiolytics (yes/no); and
- Presence of mood switch (yes/no; not included when switch was the predicted variable).
- Response to lithium (CACNG2);
- Reduced metabolism of quetiapine (CYP3A4);
- Variable metabolism of second-generation antipsychotics other than quetiapine (CYP1A2, CYP2D6);
- Lower side effects of risperidone or paliperidone (AKT1-FCHSDQ-RPTOR-DDIT4);
- Response to selective serotonin reuptake inhibitors (SSRIs) (SLC6A4, BDNF, ABCB1);
- Increased side effects of SSRIs (SLC6A4, HTR2A);
- Response to serotonin norepinephrine reuptake inhibitors (SNRIs) (ABCB1);
- Variable metabolism of SNRIs (CYP2D6);
- Response to anticonvulsants (ABCB1); and
- Increased side effects of anticonvulsants (HLA-A).
2.5.2. Clinical Outcomes
3. Results
3.1. Patient Characteristics
3.2. Primary Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.F.; Firth, J.; Vieta, E. Bipolar Disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Jin, R.; He, J.P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of role due to common physical and mental conditions: Results from the WHO World Mental Health surveys. Mol. Psychiatry 2011, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Nivoli, A.M.A.; Pacchiarotti, I.; Rosa, A.R.; Popovic, D.; Murru, A.; Valenti, M.; Bonnin, C.M.; Grande, I.; Sanchez-Moreno, J.; Vieta, E.; et al. Gender differences in a cohort study of 604 bipolar patients: The role of predominant polarity. J. Affect. Disord. 2011, 133, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M. Preventing the malignant transformation of bipolar disorder. JAMA J. Am. Med. Assoc. 2018, 319, 1197–1198. [Google Scholar] [CrossRef]
- Corponi, F.; Anmella, G.; Verdolini, N.; Pacchiarotti, I.; Samalin, L.; Popovic, D.; Azorin, J.-M.; Angst, J.; Bowden, C.L.; Mosolov, S.; et al. Symptom networks in acute depression across bipolar and major depressive disorders: A network analysis on a large, international, observational study. Eur. Neuropsychopharmacol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Grunze, H.; Vieta, E.; Goodwin, G.M.; Bowden, C.; Licht, R.W.; Möller, H.-J.; Kasper, S.; WFSBP Task Force on Treatment Guidelines for Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: Update 2012 on the long-term treatment of bipolar disorder. World J. Biol. Psychiatry 2013, 14, 154–219. [Google Scholar] [CrossRef] [Green Version]
- National Collaborating Centre for Mental Health. Bipolar Disorder the Nice Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care Updated Edition. The British Psychological Society and The Royal College of Psychiatrist. 2020. Available online: https://www.nice.org.uk/guidance/cg185 (accessed on 15 August 2021).
- Fountoulakis, K.N.; Yatham, L.; Grunze, H.; Vieta, E.; Young, A.; Blier, P.; Kasper, S.; Moeller, H.J. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 2: Review, Grading of the Evidence, and a Precise Algorithm. Int. J. Neuropsychopharmacol. 2016, 20, pyw100. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Haddad, P.M.; Ferrier, I.N.; Aronson, J.K.; Barnes, T.R.H.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.R.; Grunze, H.; et al. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef] [PubMed]
- Anmella, G.; Pacchiarotti, I.; Cubała, W.J.W.J.; Dudek, D.; Maina, G.; Thomas, P.; Vieta, E. Expert advice on the management of valproate in women with bipolar disorder at childbearing age. Eur. Neuropsychopharmacol. 2019, 29, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Tansey, K.E.; Guipponi, M.; Hu, X.; Domenici, E.; Lewis, G.; Malafosse, A.; Wendland, J.R.; Lewis, C.M.; McGuffin, P.; Uher, R. Contribution of common genetic variants to antidepressant response. Biol. Psychiatry 2013, 73, 679–682. [Google Scholar] [CrossRef]
- Smith, R.M. Advancing psychiatric pharmacogenomics using drug development paradigms. Pharmacogenomics 2017, 18, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Bousman, C.A.; Ng, C.; Berk, M. Antidepressant pharmacogenetics. Curr. Opin. Psychiatry 2014, 27, 43–51. [Google Scholar] [CrossRef]
- Pisanu, C.; Heilbronner, U.; Squassina, A. The Role of Pharmacogenomics in Bipolar Disorder: Moving Towards Precision Medicine. Mol. Diagn. Ther. 2018, 22, 409–420. [Google Scholar] [CrossRef]
- Stahl, E.A.; Breen, G.; Forstner, A.J.; McQuillin, A.; Ripke, S.; Trubetskoy, V.; Mattheisen, M.; Wang, Y.; Coleman, J.R.I.; Gaspar, H.A.; et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019, 51, 793–803. [Google Scholar] [CrossRef]
- Hou, L.; Heilbronner, U.; Degenhardt, F.; Adli, M.; Akiyama, K.; Akula, N.; Ardau, R.; Arias, B.; Backlund, L.; Banzato, C.E.; et al. Genetic variants associated with response to lithium treatment in bipolar disorder: A genome-wide association study. Lancet 2016, 387, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef]
- Hung, S.I.; Chung, W.H.; Jee, S.H.; Chen, W.C.; Chang, Y.T.; Lee, W.R.; Hu, S.L.; Wu, M.T.; Chen, G.S.; Wong, T.W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharm. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperaviciute, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I.W.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Phillips, E.J.; Sukasem, C.; Whirl-Carrillo, M.; Müller, D.J.; Dunnenberger, H.M.; Chantratita, W.; Goldspiel, B.; Chen, Y.; Carleton, B.C.; George, A.L.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amstutz, U.; Shear, N.H.; Rieder, M.J.; Hwang, S.; Fung, V.; Nakamura, H.; Connolly, M.B.; Ito, S.; Carleton, B.C. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia 2014, 55, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Barboza, A.B.; McElroy, S.L.; Veldic, M.; Singh, B.; Kung, S.; Romo-Nava, F.; Nunez, N.A.; Cabello-Arreola, A.; Coombes, B.J.; Prieto, M.; et al. Potential pharmacogenomic targets in bipolar disorder: Considerations for current testing and the development of decision support tools to individualize treatment selection. Int. J. Bipolar Disord. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-Garcia, E.; Vieta, E.; Olivares, J.M.; et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: Results of a randomized, double-blind clinical trial. BMC Psychiatry 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Wang, S.-M.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Mandelli, L.; Pae, C.-U.; Serretti, A. A Pharmacogenomic-based Antidepressant Treatment for Patients with Major Depressive Disorder: Results from an 8-week, Randomized, Single-blinded Clinical Trial. Clin. Psychopharmacol. Neurosci. 2018, 16, 469–480. [Google Scholar] [CrossRef]
- Ielmini, M.; Poloni, N.; Caselli, I.; Espadaler, J.; Tuson, M.; Grecchi, A.; Callegari, C. The utility of pharmacogenetic testing to support the treatment of bipolar disorder. Pharmgenomics Pers. Med. 2018, 11, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Callegari, C.; Isella, C.; Caselli, I.; Poloni, N.; Ielmini, M. Pharmacogenetic Tests in Reducing Accesses to Emergency Services and Days of Hospitalization in Bipolar Disorder: A 2-Year Mirror Analysis. J. Pers. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Vieta, E.; Torrent, C.; Martínez-Aran, A.; Colom, F.; Reinares, M.; Benabarre, A.; Comes, M.; Goikolea, J.M. [A user-friendly scale for the short and long term outcome of bipolar disorder: The CGI-BP-M]. Actas Españolas Psiquiatr. 2002, 30, 301–304. [Google Scholar]
- Ramos-Brieva, J.A.; Cordero-Villafafila, A. A new validation of the Hamilton Rating Scale for depression. J. Psychiatr. Res. 1988, 22, 21–28. [Google Scholar] [CrossRef]
- Rosa, A.R.; Sánchez-Moreno, J.; Martínez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.; et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin. Pract. Epidemiol. Ment. Health 2007, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 15 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 15 August 2021).
- Brown, L.; Vranjkovic, O.; Li, J.; Yu, K.; Al Habbab, T.; Johnson, H.; Brown, K.; Jablonski, M.R.; Dechairo, B. The clinical utility of combinatorial pharmacogenomic testing for patients with depression: A meta-analysis. Pharmacogenomics 2020, 21, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Corponi, F.; Fabbri, C.; Serretti, A. Pharmacogenetics and depression: A critical perspective. Psychiatry Investig. 2019, 16, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Vilches, S.; Tuson, M.; Vieta, E.; Álvarez, E.; Espadaler, J. Effectiveness of a pharmacogenetic tool at improving treatment efficacy in major depressive disorder: A meta-analysis of three clinical studies. Pharmaceutics 2019, 11, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortinguerra, S.; Sorrenti, V.; Giusti, P.; Zusso, M.; Buriani, A. Pharmacogenomic characterization in bipolar spectrum disorders. Pharmaceutics 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieta, E.; Angst, J. Bipolar disorder cohort studies: Crucial, but underfunded. Eur. Neuropsychopharmacol. 2021, 47, 31–33. [Google Scholar] [CrossRef]
- Hodges, L.M.; Markova, S.M.; Chinn, L.W.; Gow, J.M.; Kroetz, D.L.; Klein, T.E.; Altman, R.B. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharm. Genom. 2011, 21, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhr, M.; Tontsch, A.; Namendorf, C.; Ripke, S.; Lucae, S.; Ising, M.; Dose, T.; Ebinger, M.; Rosenhagen, M.; Kohli, M.; et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron 2008, 57, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Peters, E.J.; Reus, V.; Hamilton, S.P. The ABCB1 transporter gene and antidepressant response. F1000 Biol. Rep. 2009, 1, 23. [Google Scholar] [CrossRef]
- Breitenstein, B.; Scheuer, S.; Bruckl, T.M.; Meyer, J.; Ising, M.; Uhr, M.; Holsboer, F. Association of ABCB1 gene variants, plasma antidepressant concentration, and treatment response: Results from a randomized clinical study. J. Psychiatr. Res. 2016, 73, 86–95. [Google Scholar] [CrossRef]
- Bruckl, T.M.; Uhr, M. ABCB1 genotyping in the treatment of depression. Pharmacogenomics 2016, 17, 2039–2069. [Google Scholar] [CrossRef]
- Ray, A.; Tennakoon, L.; Keller, J.; Sarginson, J.E.-M.; Ryan, H.S.; Murphy, G.M.; Lazzeroni, L.C.; Trivedi, M.H.; Kocsis, J.H.; Debattista, C.; et al. ABCB1 (MDR1) predicts remission on P-gp substrates in chronic depression. Pharm. J. 2015, 15, 332–339. [Google Scholar] [CrossRef]
- Bet, P.M.; Verbeek, E.C.; Milaneschi, Y.; Straver, D.B.; Uithuisje, T.; Bevova, M.R.; Hugtenburg, J.G.; Heutink, P.; Penninx, B.W.; Hoogendijk, W.J. A common polymorphism in the ABCB1 gene is associated with side effects of PGP-dependent antidepressants in a large naturalistic Dutch cohort. Pharm. J. 2016, 16, 202–208. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: Clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2012, 165, 289–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, M.; Murayama, N.; Shimizu, M.; Shimada, T.; Guengerich, F.P.; Yamazaki, H. CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J. Toxicol. Sci. 2013, 38, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elens, L.; Van Gelder, T.; Hesselink, D.A.; Haufroid, V.; Van Schaik, R.H.N. CYP3A4*22: Promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 2013, 14, 47–62. [Google Scholar] [CrossRef]
- van der Weide, K.; van der Weide, J. The influence of the CYP3A4*22 polymorphism on serum concentration of quetiapine in psychiatric patients. J. Clin. Psychopharmacol. 2014, 34, 256–260. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Ritter, M.A.; Malagelada, C.; Bernardo, M.; Lafuente, A. Pharmacogenetic predictor of extrapyramidal symptoms induced by antipsychotics: Multilocus interaction in the mTOR pathway. Eur. Neuropsychopharmacol. 2015, 25, 51–59. [Google Scholar] [CrossRef]
- Haslemo, T.; Eikeseth, P.H.; Tanum, L.; Molden, E.; Refsum, H. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur. J. Clin. Pharmacol. 2006, 62, 1049–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, Y.; Saruwatari, J.; Yasui-Furukori, N. Meta-analysis: The effects of smoking on the disposition of two commonly used antipsychotic agents, olanzapine and clozapine. BMJ Open 2014, 4, e004216. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.F.; Ghert, M.; Simpson, A.H.R.W. Interpreting regression models in clinical outcome studies. Bone Jt. Res. 2015, 4, 152–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Characteristic | n = 76 | |
|---|---|---|
| Gender, n (%) | ||
| Female | 50 (65.78) | |
| Male | 26 (34.21) | |
| Ethnicity, n (%) | ||
| European | 70 (92.10) | |
| Asian | 1 (1.31) | |
| Hispanic | 3 (3.94) | |
| Afro-American | 1 (1.31) | |
| Others | 1 (1.31) | |
| Bipolar disorder type, n (%) | ||
| BD I | 41 (53.94) | |
| BD II | 34 (44.73) | |
| Data missing | 1 (1.31) | |
| Age at sampling (years), mean ± SD | 43.54 ± 9.08 | |
| Age of onset of bipolar disorder (years), mean ± SD | 31.16 ± 10.66 | |
| Duration of illness (years), mean ± SD | 11.91 ± 8.64 | |
| Duration of index episodes (days), mean ± SD | 76.91 ± 53.09 | |
| Number of drugs in index episode, mean ± SD | 4.54 ± 1.43 | |
| Mood switch after index episode, n (%) | 20 (26.31) | |
| Number of adverse effects, mean ± SD | 0.92 ± 1.03 | |
| Medical comorbidities, n (%) | 22 (28.94) | |
| Non psychiatric comorbidities, n (%) | 14 (18.42) | |
| Other psychiatric diagnostics, n (%) | 14 (18.42) | |
| Smoking, n (%) | 40 (52.63) | |
| Cigarettes per day, mean ± SD | 10.03 ± 12.59 | |
| Alcohol intake, n (%) | 11 (14.47) | |
| Standard drink units per day, mean ± SD | 0.34 ± 1.08 | |
| Substance use disorder, n (%) | 5 (6.58) | |
| Time of Assessment | |||
|---|---|---|---|
| Variable | Onset of the Episode | End of the Episode | Enrollment Visit |
| CGI-BP-M, mean ± SD | |||
| Overall | 3.95 ± 0.79 | 2.01 ± 0.76 | 2.01 ± 0.85 |
| Depression | 3.99 ± 0.72 | 2.04 ± 0.76 | 1.99 ± 0.87 |
| Mania | 1.39 ± 0.54 | 1.45 ± 0.60 | 1.16 ± 0.40 |
| HDRS-17, mean ± SD | na | na | 7.90 ± 6.04 |
| FAST, mean ± SD | na | na | 26.99 ± 19.04 |
| Variables | B | SD | β | t | Sig. |
|---|---|---|---|---|---|
| CGI-BP-M overall | |||||
| R2 = 0.249; F = 5.898; p-value = 4 × 10−4 | |||||
| (constant) | 1.457 | 0.150 | 9.722 | 0.000 | |
| Response to SNRIs | −0.454 | 0.205 | −0.230 | −2.215 | 0.030 |
| Smoke quantity | 0.019 | 0.007 | 0.273 | 2.629 | 0.010 |
| Treatment with anxiolytics in IE | 0.457 | 0.181 | 0.263 | 2.529 | 0.014 |
| Reduced metabolism of quetiapine | −0.288 | 0.136 | −0.221 | −2.111 | 0.038 |
| CGI-BP-M depression | |||||
| R2 = 0.262; F = 6.303; p-value = 2 × 10−4 | |||||
| (constant) | 1.405 | 0.151 | 9.292 | 0.000 | |
| Response to SNRIs | −0.472 | 0.207 | −0.235 | 2.281 | 0.026 |
| Smoke quantity | 0.020 | 0.007 | 0.290 | 2.816 | 0.006 |
| Treatment with anxiolytics in IE | 0.446 | 0.183 | 0.252 | 2.445 | 0.017 |
| Reduced metabolism of quetiapine | −0.315 | 0.138 | −0.238 | −2.292 | 0.025 |
| HDRS-17 | |||||
| R2 = 0.326; F = 8.354; p-value = 0.000 | |||||
| (constant) | -0.647 | 3.016 | −0.215 | 0.831 | |
| Response to SNRIs | −4.753 | 1.408 | −0.336 | −3.376 | 0.001 |
| Age at enrollment | 0.137 | 0.071 | 0.205 | 1.929 | 0.058 |
| Other psychiatric comorbidities | 4.314 | 1.528 | 0.281 | 2.824 | 0.006 |
| Medical comorbidities | 3.025 | 1.399 | 0.230 | 2.162 | 0.034 |
| Number of adverse effects | |||||
| R2 = 0.168; F = 6.664; p-value = 0.0023 | |||||
| (constant) | 0.731 | 0.152 | 4.809 | 0.000 | |
| Number of previous episodes | 0.032 | 0.011 | 0.336 | 2.983 | 0.004 |
| Response to SNRIs | 0.584 | 0.252 | 0.261 | 2.316 | 0.024 |
| Number of adverse effects (including suicidal ideation and mood switch) | |||||
| R2 = 0.138; F = 5.779; p-value = 0.0047 | |||||
| (constant) | 1.576 | 0.126 | 12.531 | 0.000 | |
| Lower side effects of risperidone or paliperidone | −1.115 | 0.439 | −0.278 | −2.539 | 0.013 |
| Other psychiatric comorbidities | 0.638 | 0.291 | 0.240 | 2.197 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anmella, G.; Vilches, S.; Espadaler-Mazo, J.; Murru, A.; Pacchiarotti, I.; Tuson, M.; Garriga, M.; Solé, E.; Brat, M.; Fico, G.; et al. Genetic Variations Associated with Long-Term Treatment Response in Bipolar Depression. Genes 2021, 12, 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12081259
Anmella G, Vilches S, Espadaler-Mazo J, Murru A, Pacchiarotti I, Tuson M, Garriga M, Solé E, Brat M, Fico G, et al. Genetic Variations Associated with Long-Term Treatment Response in Bipolar Depression. Genes. 2021; 12(8):1259. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12081259
Chicago/Turabian StyleAnmella, Gerard, Silvia Vilches, Jordi Espadaler-Mazo, Andrea Murru, Isabella Pacchiarotti, Miquel Tuson, Marina Garriga, Eva Solé, Mercè Brat, Giovanna Fico, and et al. 2021. "Genetic Variations Associated with Long-Term Treatment Response in Bipolar Depression" Genes 12, no. 8: 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12081259