A Flashforward Look into Solutions for Fruit and Vegetable Production
Abstract
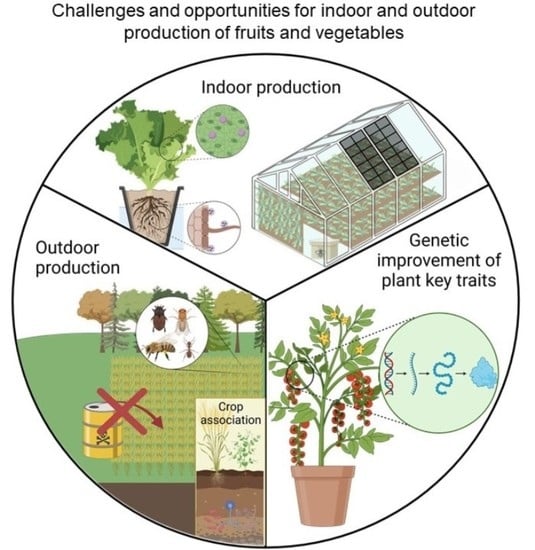
:1. Introduction
2. Solutions for Outdoor Production Systems to Reach Sustainability
2.1. Crop Migration and Adaptivity to Face Climate Change
2.2. Promoting Biodiversity
2.3. Safeguarding Pollinators
2.4. Preserving Soil Health
2.5. Preserving Water Availability and Quality
3. Solution in Indoor Production Systems to Reach Sustainability
3.1. Needs for New Sustainable Indoor Growing Systems
3.2. High-Tech Indoor Agriculture
3.3. New Crop Traits for Indoor Farming
4. Genetic Improvement of Cultivated Fruits and Vegetables
4.1. Improving Plant Defense Mechanisms
4.2. Improving Plant Nutrition Mechanisms
4.3. Growth Optimization for Space-Limited Environments
4.4. Improving Flowering and Fruit Set
4.5. Parthenocarpic Crops as a Solution for Both Outdoor and Indoor Growing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, J.; Sato, M.; Ruedy, R. Perception of climate change. Proc. Natl. Acad. Sci. USA 2012, 109, E2415–E2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Available online: https://www.fao.org/faostat/fr/#data/ET (accessed on 27 October 2021).
- Sévellec, F.; Drijfhout, S.S. A novel probabilistic forecast system predicting anomalously warm 2018–2022 reinforcing the long-term global warming trend. Nat. Commun. 2018, 9, 3024. [Google Scholar] [CrossRef]
- Arnell, N.W.; Gosling, S.N. The impacts of climate change on river flood risk at the global scale. Clim. Chang. 2016, 134, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Angel, S.; Blei, A.M.; Parent, J.; Lamson-Hall, P.; Galarza Sánchez, N. Atlas of Urban Expansion—2016 Edition, Volume 1: Areas and Densities; New York University: New York, NY, USA; UN-Habitat: Nairobi, Kenya; Lincoln Institute of Land Policy: Cambridge, MA, USA, 2016. [Google Scholar]
- United Nations; Department of Economic and Social Affairs. Population Division. In World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; ISBN 978-92-1-148316-1. [Google Scholar]
- Ritchie, H.; Roser, M. Land Use. Available online: https://ourworldindata.org/land-use (accessed on 11 March 2021).
- Buckley, L.B.; Huey, R.B. Temperature extremes: Geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Chang. Biol. 2016, 22, 3829–3842. [Google Scholar] [CrossRef]
- King, M.; Altdorff, D.; Li, P.; Galagedara, L.; Holden, J.; Unc, A. Northward shift of the agricultural climate zone under 21st-century global climate change. Sci. Rep. 2018, 8, 7904. [Google Scholar] [CrossRef] [Green Version]
- Seguin, B. Adaptation des systèmes de production agricole au changement climatique. C. R. Geosci. 2003, 335, 569–575. [Google Scholar] [CrossRef]
- Rising, J.; Devineni, N. Crop switching reduces agricultural losses from climate change in the United States by half under RCP 8.5. Nat. Commun. 2020, 11, 4991. [Google Scholar] [CrossRef]
- Davis, K.F.; Rulli, M.C.; Seveso, A.; D’Odorico, P. Increased food production and reduced water use through optimized crop distribution. Nat. Geosci. 2017, 10, 919–924. [Google Scholar] [CrossRef]
- Dias, C.; Mendes, L. Protected Designation of Origin (PDO), Protected Geographical Indication (PGI) and Traditional Speciality Guaranteed (TSG): A bibiliometric analysis. Food Res. Int. Ott. Ont. 2018, 103, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.E.; Wilson, R.J.; Maclean, I.M.D. Climate change impacts and adaptive strategies: Lessons from the grapevine. Glob. Chang. Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Y.; Zhang, P.; Shang, Y. The potential global distribution and dynamics of wheat under multiple climate change scenarios. Sci. Total Environ. 2019, 688, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, J.A.; Roth, C.L.; Mushet, D.M. Modeling effects of crop production, energy development and conservation-grassland loss on avian habitat. PLoS ONE 2019, 14, e0198382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerlekar, A.N.; Veldman, J.W. High plant diversity and slow assembly of old-growth grasslands. Proc. Natl. Acad. Sci. USA 2020, 117, 18550–18556. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Land use competition for production of food and liquid biofuels: An analysis of the arguments in the current debate. Renew. Energy 2010, 35, 14–22. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann. Bot. 2012, 110, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wang, G.; Wu, Y.; Shi, Y.; Feng, Y.; Cao, F. Ginkgo agroforestry practices alter the fungal community structures at different soil depths in Eastern China. Environ. Sci. Pollut. Res. Int. 2019, 26, 21253–21263. [Google Scholar] [CrossRef]
- Abbas, F.; Hammad, H.M.; Fahad, S.; Cerdà, A.; Rizwan, M.; Farhad, W.; Ehsan, S.; Bakhat, H.F. Agroforestry: A sustainable environmental practice for carbon sequestration under the climate change scenarios—A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 11177–11191. [Google Scholar] [CrossRef] [PubMed]
- Caron, B.O.; Pinheiro, M.V.M.; Korcelski, C.; Schwerz, F.; Elli, E.F.; Sgarbossa, J.; Tibolla, L.B. Agroforestry systems and understory harvest management: The impact on growth and productivity of dual-purpose wheat. An. Acad. Bras. Cienc. 2019, 91, e20180667. [Google Scholar] [CrossRef]
- Potts, S.G. The Assessment Report on Pollinators, Pollination and Food Production: Summary for Policymakers; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; ISBN 978-92-807-3568-0. [Google Scholar]
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Miller-Rushing, A.J.; Primack, R.B. Global Warming and Flowering Times in Thoreau’s Concord: A Community Perspective. Ecology 2008, 89, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, N.E.; Ives, A.R. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology 2012, 93, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu. Rev. Èntomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, P.; Tscharntke, T.; Tylianakis, J.M.; Steffan-Dewenter, I. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B Biol. Sci. 2008, 275, 2283–2291. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Garratt, M.P.D.; Powney, G.D.; Shaw, R.F.; Osborne, J.L.; Soroka, J.; Lindström, S.A.M.; Stanley, D.; Ouvrard, P.; Edwards, M.E.; et al. Meta-analysis reveals that pollinator functional diversity and abundance enhance crop pollination and yield. Nat. Commun. 2019, 10, 1481. [Google Scholar] [CrossRef] [Green Version]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Sáez, A.; Aizen, M.A.; Fijen, T.; Bartomeus, I. Crop pollination management needs flower-visitor monitoring and target values. J. Appl. Ecol. 2020, 57, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkaerne, S.; et al. Agricultural Policies Exacerbate Honeybee Pollination Service Supply-Demand Mismatches Across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.W.; Debinski, D.M.; Scavo, N.A.; Lange, C.J.; Delaney, J.T.; Moranz, R.A.; Miller, J.R.; Engle, D.M.; Toth, A.L. Bee Abundance and Nutritional Status in Relation to Grassland Management Practices in an Agricultural Landscape. Environ. Èntomol. 2016, 45, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Hostyánszki, A.; Espíndola, A.; Vanbergen, A.J.; Settele, J.; Kremen, C.; Dicks, L.V. Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol. Lett. 2017, 20, 673–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Corzo Remigio, A.; Chaney, R.L.; Baker, A.J.M.; Edraki, M.; Erskine, P.D.; Echevarria, G.; van der Ent, A. Phytoextraction of high value elements and contaminants from mining and mineral wastes: Opportunities and limitations. Plant Soil 2020, 449, 11–37. [Google Scholar] [CrossRef]
- Yang, X.; Bento, C.P.M.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 338–347. [Google Scholar] [CrossRef]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Martinez, R.E.; Sharma, P.; Kappler, A. Surface binding site analysis of Ca2+-homoionized clay–humic acid complexes. J. Colloid Interface Sci. 2010, 352, 526–534. [Google Scholar] [CrossRef]
- Xu, Q.; Duan, D.; Cai, Q.; Shi, J. Influence of Humic Acid on Pb Uptake and Accumulation in Tea Plants. J. Agric. Food Chem. 2018, 66, 12327–12334. [Google Scholar] [CrossRef] [PubMed]
- Paleckiene, R.; Navikaite, R.; Slinksiene, R. Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability 2021, 13, 6354. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated Use of Humic Acid and Plant Growth Promoting Rhizobacteria to Ensure Higher Potato Productivity in Sustainable Agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Antonietti, M. Artificial Humic Acids: Sustainable Materials against Climate Change. Adv. Sci. 2020, 7, 1902992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindo, K.; Olivares, F.L.; da Paixao Malcher, D.J.; Angel Sánchez-Monedero, M.; Kempenaar, C.; Canellas, L.P. From Lab to Field: Role of Humic Substances Under Open-Field and Greenhouse Conditions as Biostimulant and Biocontrol Agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, F.; Wang, Z.; Xing, B. The role of biochars in sustainable crop production and soil resiliency. J. Exp. Bot. 2020, 71, 520–542. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- He, Z.; Zhang, M.; Zhao, A.; Olanya, O.M.; Larkin, R.P.; Honeycutt, C.W. Quantity and Nature of Water-Extractable Organic Matter from Sandy Loam Soils with Potato Cropping Management. Agric. Environ. Lett. 2016, 1, 160023. [Google Scholar] [CrossRef]
- Guimarães Júnnyor, W.D.S.; Diserens, E.; De Maria, I.C.; Araujo-Junior, C.F.; Farhate, C.V.V.; de Souza, Z.M. Prediction of soil stresses and compaction due to agricultural machines in sugarcane cultivation systems with and without crop rotation. Sci. Total Environ. 2019, 681, 424–434. [Google Scholar] [CrossRef]
- Jian, J.; Du, X.; Stewart, R.D. Quantifying cover crop effects on soil health and productivity. Data Brief 2020, 29, 105376. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P.; Mench, M.; Álvarez-López, V.; Bert, V.; Dimitriou, I.; Friesl-Hanl, W.; Herzig, R.; Olga Janssen, J.; Kolbas, A.; Müller, I.; et al. Agronomic Practices for Improving Gentle Remediation of Trace Element-Contaminated Soils. Int. J. Phytoremediat. 2015, 17, 1005–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henneron, L.; Bernard, L.; Hedde, M.; Pelosi, C.; Villenave, C.; Chenu, C.; Bertrand, M.; Girardin, C.; Blanchart, E. Fourteen years of evidence for positive effects of conservation agriculture and organic farming on soil life. Agron. Sustain. Dev. 2015, 35, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Javaria, S.; Khan, M.Q. Impact of integrated nutrient management on tomato yield quality and soil environment. J. Plant Nutr. 2010, 34, 140–149. [Google Scholar] [CrossRef]
- Kumari, R.; Kundu, M.; Das, A.; Rakshit, R.; Sahay, S.; Sengupta, S.; Ahmad, M.F. Long-Term Integrated Nutrient Management Improves Carbon Stock and Fruit Yield in a Subtropical Mango (Mangifera indica L.) Orchard. J. Soil Sci. Plant Nutr. 2020, 20, 725–737. [Google Scholar] [CrossRef]
- Schrama, M.; de Haan, J.J.; Kroonen, M.; Verstegen, H.; Van der Putten, W.H. Crop yield gap and stability in organic and conventional farming systems. Agric. Ecosyst. Environ. 2018, 256, 123–130. [Google Scholar] [CrossRef]
- Bergström, L.; Kirchmann, H. Are the claimed benefits of organic agriculture justified? Nat. Plants 2016, 2, 16099. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; Scialabba, N.E.-H.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef]
- Connor, D.J. Organically grown crops do not a cropping system make and nor can organic agriculture nearly feed the world. Field Crop. Res. 2013, 144, 145–147. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A. Building plant microbiome vault: A future biotechnological resource. Symbiosis 2019, 77, 1–8. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Steering soil microbiome to enhance soil system resilience. Crit. Rev. Microbiol. 2019, 45, 743–753. [Google Scholar] [CrossRef]
- Schilling, J.; Hertig, E.; Tramblay, Y.; Scheffran, J. Climate change vulnerability, water resources and social implications in North Africa. Reg. Environ. Chang. 2020, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Hänsel, S.; Ustrnul, Z.; Łupikasza, E.; Skalak, P. Assessing seasonal drought variations and trends over Central Europe. Adv. Water Resour. 2019, 127, 53–75. [Google Scholar] [CrossRef]
- Pfleiderer, P.; Schleussner, C.-F.; Kornhuber, K.; Coumou, D. Summer weather becomes more persistent in a 2 °C world. Nat. Clim. Chang. 2019, 9, 666–671. [Google Scholar] [CrossRef]
- Leng, G.; Tang, Q.; Rayburg, S. Climate change impacts on meteorological, agricultural and hydrological droughts in China. Glob. Planet. Chang. 2015, 126, 23–34. [Google Scholar] [CrossRef]
- Konapala, G.; Mishra, A.K.; Wada, Y.; Mann, M.E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 2020, 11, 3044. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—Past, present, and future. Theor. Appl. Climatol. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
- Gbode, I.E.; Diro, G.T.; Intsiful, J.D.; Dudhia, J. Current Conditions and Projected Changes in Crop Water Demand, Irrigation Requirement, and Water Availability over West Africa. Atmosphere 2022, 13, 1155. [Google Scholar] [CrossRef]
- Crop Water Demand—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/water-requirement-2/assessment (accessed on 20 September 2022).
- Zhou, T.; Wu, P.; Sun, S.; Li, X.; Wang, Y.; Luan, X. Impact of Future Climate Change on Regional Crop Water Requirement—A Case Study of Hetao Irrigation District, China. Water 2017, 9, 429. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Food Legume Production. PLoS ONE 2015, 10, e0127401. [Google Scholar] [CrossRef] [Green Version]
- Wein, A.; Mitchell, D.; Peters, J.; Rowden, J.; Tran, J.; Corsi, A.; Dinitz, L. Agricultural Damages and Losses from ARkStorm Scenario Flooding in California. Nat. Hazards Rev. 2016, 17, 15001. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Chen, H. Investigating Flood Impact on Crop Production under a Comprehensive and Spatially Explicit Risk Evaluation Framework. Agriculture 2022, 12, 484. [Google Scholar] [CrossRef]
- Li, J.; Fei, L.; Li, S.; Xue, C.; Shi, Z.; Hinkelmann, R. Development of “water-suitable” agriculture based on a statistical analysis of factors affecting irrigation water demand. Sci. Total Environ. 2020, 744, 140986. [Google Scholar] [CrossRef]
- Rosa, L. Adapting agriculture to climate change via sustainable irrigation: Biophysical potentials and feedbacks. Environ. Res. Lett. 2022, 17, 063008. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health: Nutrition Reviews©, Vol. 68, No. 8. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A Review on Potential Plant-Based Water Stress Indicators for Vegetable Crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Saadi, S.; Todorovic, M.; Tanasijevic, L.; Pereira, L.S.; Pizzigalli, C.; Lionello, P. Climate change and Mediterranean agriculture: Impacts on winter wheat and tomato crop evapotranspiration, irrigation requirements and yield. Agric. Water Manag. 2015, 147, 103–115. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane technology for water production in agricultura: Desalina-tion and wastewater reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Vema, V.K.; Kurian, C.; Sudheer, K.P. Improving the crop productivity in rainfed areas with water harvesting structures and deficit irrigation strategies. J. Hydrol. 2020, 586, 124818. [Google Scholar] [CrossRef]
- Assouline, S. A Simple Method to Design Irrigation Rate and Duration and Improve Water Use Efficiency. Water Resour. Res. 2019, 55, 6295–6301. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.-M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, D.; Öztekin, G.B.; Tüzel, Y.; Brückner, B.; Krumbein, A. Rootstocks can enhance tomato growth and quality characteristics at low potassium supply. Sci. Hortic. 2013, 149, 70–79. [Google Scholar] [CrossRef]
- Puy, A.; Borgonovo, E.; Lo Piano, S.; Levin, S.A.; Saltelli, A. Irrigated areas drive irrigation water withdrawals. Nat. Commun. 2021, 12, 4525. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2015, 36, 3. [Google Scholar] [CrossRef] [Green Version]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef]
- Sharma, S.P.; Leskovar, D.I.; Crosby, K.M.; Volder, A.; Ibrahim, A.M.H. Root growth, yield, and fruit quality responses of reticulatus and inodorus melons (Cucumis melo L.) to deficit subsurface drip irrigation. Agric. Water Manag. 2014, 136, 75–85. [Google Scholar] [CrossRef]
- Afzaal, H.; Farooque, A.A.; Abbas, F.; Acharya, B.; Esau, T. Precision Irrigation Strategies for Sustainable Water Budgeting of Potato Crop in Prince Edward Island. Sustainability 2020, 12, 2419. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, S. Water and Energy Scarcity for Agriculture: Is Irrigation Modernization the Answer? Irrig. Drain. 2017, 66, 34–44. [Google Scholar] [CrossRef]
- Gunarathna, M.H.J.P.; Sakai, K.; Nakandakari, T.; Kazuro, M.; Onodera, T.; Kaneshiro, H.; Uehara, H.; Wakasugi, K. Optimized Subsurface Irrigation System (OPSIS): Beyond Traditional Subsurface Irrigation. Water 2017, 9, 599. [Google Scholar] [CrossRef]
- Kashyap, P.K.; Kumar, S.; Jaiswal, A.; Prasad, M.; Gandomi, A.H. Towards Precision Agriculture: IoT-Enabled Intelligent Irrigation Systems Using Deep Learning Neural Network. IEEE Sens. J. 2021, 21, 17479–17491. [Google Scholar] [CrossRef]
- Bwambale, E.; Abagale, F.K.; Anornu, G.K. Smart irrigation monitoring and control strategies for improving water use efficiency in precision agriculture: A review. Agric. Water Manag. 2022, 260, 107324. [Google Scholar] [CrossRef]
- Adeyemi, O.; Grove, I.; Peets, S.; Norton, T. Advanced Monitoring and Management Systems for Improving Sustainability in Precision Irrigation. Sustainability 2017, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Sabir, N.; Singh, B. Protected Cultivation of Vegetables in Global Arena: A Review. Indian J. Agric. Sci. 2013, 83, 123–135. [Google Scholar]
- Papasolomontos, A.; Baudoin, W.; Lutaladio, N.; Castilla, N.; Baeza, E.; Montero, J.I.; Teitel, M.; Lopez, J.C.; Kacira, M.; Kittas, C.; et al. Good Agricultural Practices for Greenhouse Vegetable Crops Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; ISBN 978-92-5-107649-1. [Google Scholar]
- Golzar, F.; Heeren, N.; Hellweg, S.; Roshandel, R. A comparative study on the environmental impact of greenhouses: A probabilistic approach. Sci. Total Environ. 2019, 675, 560–569. [Google Scholar] [CrossRef]
- Parlato, M.C.M.; Valenti, F.; Porto, S.M. Covering plastic films in greenhouses system: A GIS-based model to improve post use suistainable management. J. Environ. Manag. 2020, 263, 110389. [Google Scholar] [CrossRef]
- Huang, S. Global Trade Patterns in Fruits and Vegetables. SSRN Electron. J. 2004. [Google Scholar] [CrossRef] [Green Version]
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban agriculture in the developing world: A review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Liesa, J.; Toboso-Chavero, S.; Mendoza Beltran, A.; Cuerva, E.; Gallo, E.; Gassó-Domingo, S.; Josa, A. Building-integrated agriculture: Are we shifting environmental impacts? An environmental assessment and structural improvement of urban greenhouses. Resour. Conserv. Recycl. 2021, 169, 105526. [Google Scholar] [CrossRef]
- Jing, R.; Hastings, A.; Guo, M. Sustainable Design of Urban Rooftop Food-Energy-Land Nexus. iScience 2020, 23, 101743. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical farming: A summary of approaches to growing skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Shaikh, S.A.; Lakhiar, I.A.; Qureshi, W.A.; Solangi, K.A.; Chandio, F.A. Potato production in aeroponics: An emerging food growing system in sustainable agriculture forfood security. Chil. J. Agric. Res. 2020, 80, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Bailey, D.S.; Ferrarezi, R.S. Valuation of vegetable crops produced in the UVI Commercial Aquaponic System. Aquac. Rep. 2017, 7, 77–82. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.M.; Verma, A.K.; Prakash, C.; Krishna, H.; Prakash, S.; Kumar, A. Utilization of Inland saline underground water for bio-integration of Nile tilapia (Oreochromis niloticus) and spinach (Spinacia oleracea). Agric. Water Manag. 2019, 222, 154–160. [Google Scholar] [CrossRef]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Urrestarazu, L.; Lobillo-Eguíbar, J.; Fernández-Cañero, R.; Fernández-Cabanás, V.M. Suitability and optimization of FAO’s small-scale aquaponics systems for joint production of lettuce (Lactuca sativa) and fish (Carassius auratus). Aquac. Eng. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Delaide, B.; Teerlinck, S.; Decombel, A.; Bleyaert, P. Effect of wastewater from a pikeperch (Sander lucioperca L.) recirculated aquaculture system on hydroponic tomato production and quality. Agric. Water Manag. 2019, 226, 5814. [Google Scholar] [CrossRef]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the roots of aeroponic indoor farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Fraternale, D.; Donati Zeppa, S.; Verardo, G.; Gorassini, A.; Carrabs, V.; Albertini, M.C.; Sestili, P. Yield, Characterization, and Possible Exploitation of Cannabis Sativa L. Roots Grown under Aeroponics Cultivation. Molecules 2021, 26, 4889. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 253875. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Ay, C.; Kan, J.-C.; Lee, M.-T. The Effect of Radiative Cooling on Reducing the Temperature of Greenhouses. Mater. Basel Switz. 2018, 11, 1166. [Google Scholar] [CrossRef] [Green Version]
- Peña, A.; Rovira-Val, M.R.; Mendoza, J.M.F. Life cycle cost analysis of tomato production in innovative urban agriculture systems. J. Clean. Prod. 2022, 367, 133037. [Google Scholar] [CrossRef]
- Sanjuan-Delmás, D.; Llorach-Massana, P.; Nadal, A.; Ercilla-Montserrat, M.; Muñoz, P.; Ignacio Montero, J.; Josa, A.; Gabarrell, X.; Rieradevall, J. Environmental assessment of an integrated rooftop greenhouse for food production in cities. J. Clean. Prod. 2018, 177, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, X.; Yan, K.; Gong, P. A Monitoring System for Vegetable Greenhouses based on a Wireless Sensor Network. Sensors 2010, 10, 8963–8980. [Google Scholar] [CrossRef]
- Liang, M.-H.; He, Y.-F.; Chen, L.-J.; Du, S.-F. Greenhouse environment dynamic monitoring system based on WIFI. IFAC-Pap. 2018, 51, 736–740. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of Nano-based Smart Pesticide Formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Karimi, M.; Teixeira Da Silva, J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2019, 100, 25–31. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a new sustainable approach for controlling crop diseases and increasing agricultural production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Hemming, S.; De Zwart, F.; Elings, A.; Petropoulou, A.; Righini, I. Cherry Tomato Production in Intelligent Greenhouses—Sensors and AI for Control of Climate, Irrigation, Crop Yield, and Quality. Sensors 2020, 20, 6430. [Google Scholar] [CrossRef] [PubMed]
- Too, E.C.; Yujian, L.; Njuki, S.; Yingchun, L. A comparative study of fine-tuning deep learning models for plant disease identification. Comput. Electron. Agric. 2019, 161, 272–279. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Kong, J.-L.; Jin, X.-B.; Wang, X.-Y.; Su, T.-L.; Zuo, M. CropDeep: The Crop Vision Dataset for Deep-Learning-Based Classification and Detection in Precision Agriculture. Sensors 2019, 19, 1058. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, J.; Tan, Q.; Peters, A.L.; Yang, C. Global status of recycling waste solar panels: A review. Waste Manag. 2018, 75, 450–458. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strat. Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental impacts of solar photovoltaic systems: A critical review of recent progress and future outlook. Sci. Total Environ. 2021, 759, 143528. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Yasmeen, F.; Komatsu, S. Nanoparticles: Synthesis, Morphophysiological Effects, and Proteomic Responses of Crop Plants. Int. J. Mol. Sci. 2020, 21, 3056. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, E.; Drobne, D. Nanomaterials in Plants: A Review of Hazard and Applications in the Agri-Food Sector. Nanomater. Basel Switz. 2019, 9, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; White, J.C.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Runkle, E. Crops Suitable for Vertical Farming. Available online: https://gpnmag.com/article/crops-suitable-for-vertical-farming/ (accessed on 11 February 2021).
- Kwon, C.-T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Antúnez, L.; Ares, G.; Swaney-Stueve, M.; Jin, D.; Harker, F.R. Quality perceptions regarding external appearance of apples: Insights from experts and consumers in four countries. Postharvest Biol. Technol. 2018, 146, 99–107. [Google Scholar] [CrossRef]
- Tarancón, P.; Tárrega, A.; González, M.; Besada, C. External Quality of Mandarins: Influence of Fruit Appearance Characteristics on Consumer Choice. Foods 2021, 10, 2188. [Google Scholar] [CrossRef]
- Normann, A.; Röding, M.; Wendin, K. Sustainable Fruit Consumption: The Influence of Color, Shape and Damage on Consumer Sensory Perception and Liking of Different Apples. Sustainability 2019, 11, 4626. [Google Scholar] [CrossRef]
- Gubbuk, H.; Gunes, E.; Güven, D. Comparison of open-field and protected banana cultivation for some morphological and yield features under subtropical conditions. Acta Hortic. 2018, 1196, 173–178. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Amico, E.; Caruso, G. Effects of production system and transplanting time on yield, quality and antioxidant content of organic winter squash (Cucurbita moschata Duch.). Sci. Hortic. 2015, 183, 136–143. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- He, L.-H.; Chen, J.-Y.; Kuang, J.-F.; Lu, W.-J. Expression of three sHSP genes involved in heat pretreatment-induced chilling tolerance in banana fruit. J. Sci. Food Agric. 2012, 92, 1924–1930. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, F.; Wen, J.; Wu, Z. Overexpression of Solanum habrochaites microRNA319d (sha-miR319d) confers chilling and heat stress tolerance in tomato (S. lycopersicum). BMC Plant Biol. 2019, 19, 214. [Google Scholar] [CrossRef]
- Huo, L.; Sun, X.; Guo, Z.; Jia, X.; Che, R.; Sun, Y.; Zhu, Y.; Wang, P.; Gong, X.; Ma, F. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-F.; Liu, J.-K.; Yang, F.-M.; Zhang, G.-Y.; Wang, D.; Zhang, L.; Ou, Y.-B.; Yao, Y.-A. The WRKY transcription factor WRKY8 promotes resistance to pathogen infection and mediates drought and salt stress tolerance in Solanum lycopersicum. Physiol. Plant. 2020, 168, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, B.; Xu, X.; Chen, H.; Zou, L.; Chen, G.; Cao, B.; Chen, C.; Lei, J. Overexpression of AtEDT1/HDG11 in Chinese Kale (Brassica oleracea var. alboglabra) Enhances Drought and Osmotic Stress Tolerance. Front. Plant Sci. 2016, 7, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-Y.; Lai, L.-D.; Tong, S.-M.; Li, Q.-L. Constitutive and salt-inducible expression of SlBADH gene in transgenic tomato (Solanum lycopersicum L. cv. Micro-Tom) enhances salt tolerance. Biochem. Biophys. Res. Commun. 2013, 432, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ding, Z.; Tie, W.; Yan, Y.; Liu, Y.; Wu, C.; Liu, J.; Wang, J.; Peng, M.; Xu, B.; et al. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017, 7, 43007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, S.L.; Stoddard, F.L.; Ortiz, R. Genomic-based root plasticity to enhance abiotic stress adaptation and edible yield in grain crops. Plant Sci. 2020, 295, 10365. [Google Scholar] [CrossRef]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]
- Alarcón, A.L.; Gómez-Bellot, M.J.; Bernabe, A.J.; Calvo, G.; Fernández Martín, F. Changes in root architecture and productivity of melon (Cucumis melo L. cv. Hispano Nunhems) promoted by Glomus iranicum var. tenuihypharum. J. Hortic. Sci. Biotechnol. 2020, 95, 364–373. [Google Scholar] [CrossRef]
- Rongsawat, T.; Peltier, J.-B.; Boyer, J.-C.; Véry, A.-A.; Sentenac, H. Looking for Root Hairs to Overcome Poor Soils. Trends Plant Sci. 2021, 26, 83–94. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [Green Version]
- Morales-Navarro, S.; Pérez-Díaz, R.; Ortega, A.; de Marcos, A.; Mena, M.; Fenoll, C.; González-Villanueva, E.; Ruiz-Lara, S. Overexpression of a SDD1-Like Gene from Wild Tomato Decreases Stomatal Density and Enhances Dehydration Avoidance in Arabidopsis and Cultivated Tomato. Front. Plant Sci. 2018, 9, 940. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, Y.; Tamoi, M.; Sakuyama, H.; Maruta, T.; Ashida, H.; Yokota, A.; Shigeoka, S. Generation of transplastomic lettuce with enhanced growth and high yield. GM Crops 2010, 1, 322–326. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Kätterer, T.; Bolinder, M.A.; Berglund, K.; Kirchmann, H. Strategies for carbon sequestration in agricultural soils in northern Europe. Acta Agric. Scand. Sect. A—Anim. Sci. 2012, 62, 181–198. [Google Scholar] [CrossRef]
- Harman, G.E.; Uphoff, N. Symbiotic Root-Endophytic Soil Microbes Improve Crop Productivity and Provide Environmental Benefits. Scientifica 2019, 2019, 9106395. [Google Scholar] [CrossRef] [Green Version]
- Van De Velde, K.; Thomas, S.G.; Heyse, F.; Kaspar, R.; Van Der Straeten, D.; Rohde, A. N-terminal truncated RHT-1 proteins generated by translational reinitiation cause semi-dwarfing of wheat Green Revolution alleles. Mol. Plant 2021, 14, 679–687. [Google Scholar] [CrossRef]
- Chaïb, J.; Torregrosa, L.; MacKenzie, D.; Corena, P.; Bouquet, A.; Thomas, M.R. The grape microvine—A model system for rapid forward and reverse genetics of grapevines. Plant J. 2010, 62, 1083–1092. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Zhu, H.; Zhang, X.; Yan, W.; Xu, N.; Liu, D.; Hu, J.; Wu, Y.; Weng, Y.; et al. Melon short internode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling. Hortic. Res. 2020, 7, 202. [Google Scholar] [CrossRef]
- Guo, F.; Ma, J.; Hou, L.; Shi, S.; Sun, J.; Li, G.; Zhao, C.; Xia, H.; Zhao, S.; Wang, X.; et al. Transcriptome profiling provides insights into molecular mechanism in Peanut semi-dwarf mutant. BMC Genom. 2020, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.M.; Gleave, A.P.; Allan, A.C. Mutagenesis of kiwifruit CENTRORADIALIS-like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobson, E.M.; Johnston, R.E.; Oiestad, A.J.; Martin, J.M.; Giroux, M.J. The Impact of the Wheat Rht-B1b Semi-Dwarfing Allele on Photosynthesis and Seed Development Under Field Conditions. Front. Plant Sci. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, J.L.; Ortega, R. Genetic control of flowering time in legumes. Front. Plant Sci. 2015, 6, 207. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Z.; Zhang, C.; Zhang, H.; Li, X.; Wen, C.; Liang, Y. Molecular cloning, expression analysis, and subcellular localization of FLOWERING LOCUS T (FT) in carrot (Daucus carota L.). Mol. Breed. 2017, 37, 149. [Google Scholar] [CrossRef]
- Palatty Allesh, S.; Varma, S.; Reshmi, K.S.; Aswathi, K.; Megha, P.P.; Jasna, T.V.; Nikhila Reshmi, M.V.; Sibisha, V.C. Effect of flower sex ratio on fruit set in pumpkin (Cucurbita maxima). Sci. Hortic. 2019, 246, 1005–1008. [Google Scholar] [CrossRef]
- Jahed, K.R.; Hirst, P.M. Pollen Tube Growth and Fruit Set in Apple. HortScience 2017, 52, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Radunić, M.; Jazbec, A.; Ercisli, S.; Čmelik, Z.; Ban, S.G. Pollen-pistil interaction influence on the fruit set of sweet cherry. Sci. Hortic. 2017, 224, 358–366. [Google Scholar] [CrossRef]
- Gustafson, F.G. Parthenocarpy: Natural and artificial. Bot. Rev. 1942, 8, 599–654. [Google Scholar] [CrossRef]
- Rylski, I.; Aloni, B. Parthenocarpic Fruit Set and Development in Cucurbitaceae and Solanaceae under Protected Cultivation in Mild Winter Climate. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 1 May 1991; Volume 287, pp. 117–126. [Google Scholar]
- Picarella, M.E.; Mazzucato, A. The Occurrence of Seedlessness in Higher Plants; Insights on Roles and Mechanisms of Parthenocarpy. Front. Plant Sci. 2019, 9, 1997. [Google Scholar] [CrossRef] [Green Version]
- Clepet, C.; Devani, R.S.; Boumlik, R.; Hao, Y.; Morin, H.; Marcel, F.; Verdenaud, M.; Mania, B.; Brisou, G.; Citerne, S.; et al. The miR166–SlHB15A regulatory module controls ovule development and parthenocarpic fruit set under adverse temperatures in tomato. Mol. Plant 2021, 14, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Ren, N.; Wang, J.; Xu, Q.; Chen, X.; Qi, X. Effects of exogenous application of CPPU, NAA and GA 4+7 on parthenocarpy and fruit quality in cucumber (Cucumis sativus L.). Food Chem. 2018, 243, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Wu, T.; Liu, H.; Wang, H.; Zhang, H.; Zhao, G.; Wen, Y.; Shi, Q.; Xu, L.; Wang, Z. CPPU may induce gibberellin-independent parthenocarpy associated with PbRR9 in ‘Dangshansu’ pear. Hortic. Res. 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Galimba, K.D.; Bullock, D.G.; Dardick, C.; Liu, Z.; Callahan, A.M. Gibberellic acid induced parthenocarpic ‘Honeycrisp’ apples (Malus domestica) exhibit reduced ovary width and lower acidity. Hortic. Res. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslop-Harrison, J.S.; Schwarzacher, T. Domestication, Genomics and the Future for Banana. Ann. Bot. 2007, 100, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorguet, B.; Van Heusden, A.W.; Lindhout, P. Parthenocarpic Fruit Development in Tomato. Plant Biol. 2005, 7, 131–139. [Google Scholar] [CrossRef]
- Mezzetti, B.; Landi, L.; Pandolfini, T.; Spena, A. The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol. 2004, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Acciarri, N.; Restaino, F.; Vitelli, G.; Perrone, D.; Zottini, M.; Pandolfini, T.; Spena, A.; Rotino, G.L. Genetically modified parthenocarpic eggplants: Improved fruit productivity under both greenhouse and open field cultivation. BMC Biotechnol. 2002, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Rotino, G.L.; Acciarri, N.; Sabatini, E.; Mennella, G.; Lo Scalzo, R.; Maestrelli, A.; Molesini, B.; Pandolfini, T.; Scalzo, J.; Mezzetti, B.; et al. Open field trial of genetically modified parthenocarpic tomato: Seedlessness and fruit quality. BMC Biotechnol. 2005, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, Y.; Ezura, K.; Hu, J.; Okabe, Y.; Bénard, C.; Prodhomme, D.; Gibon, Y.; Sun, T.-P.; Ezura, H.; Ariizumi, T. Identification and functional study of a mild allele of SlDELLA gene conferring the potential for improved yield in tomato. Sci. Rep. 2018, 8, 12043. [Google Scholar] [CrossRef] [Green Version]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef]
- Spena, A.; Rotino, G.L. Parthenocarpy. In Current Trends in the Embryology of Angiosperms; Bhojwani, S.S., Soh, W.-Y., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 435–450. ISBN 978-94-017-1203-3. [Google Scholar]
- Pandolfini, T.; Rotino, G.L.; Camerini, S.; Defez, R.; Spena, A. Optimisation of transgene action at the post-transcriptional level: High quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnol. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.; Maynard, E.T.; Aly, B.; Zakes, J.; Egel, D.S.; Ingwell, L.L. Parthenocarpic Cucumber Cultivar Evaluation in High-tunnel Production. HortTechnology 2019, 29, 634–642. [Google Scholar] [CrossRef]
- dos Santos, R.C.; Nietsche, S.; Pereira, M.C.T.; Ribeiro, L.M.; Mercadante-Simões, M.O.; Carneiro dos Santos, B.H. Atemoya fruit development and cytological aspects of GA3-induced growth and parthenocarpy. Protoplasma 2019, 256, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ezura, K.; Lee, J.; Ariizumi, T.; Ezura, H. Genetic engineering of parthenocarpic tomato plants using transient SlIAA9 knockdown by novel tissue-specific promoters. Sci. Rep. 2019, 9, 18871. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.-L.; Dong, Y.-H.; Morris, B.A.M. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc. Natl. Acad. Sci. USA 2001, 98, 1306–1311. [Google Scholar] [CrossRef]
- Wada, M.; Oshino, H.; Tanaka, N.; Mimida, N.; Moriya-Tanaka, Y.; Honda, C.; Hanada, T.; Iwanami, H.; Komori, S. Expression and functional analysis of apple MdMADS13 on flower and fruit formation. Plant Biotechnol. 2018, 35, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Sardos, J.; Rouard, M.; Hueber, Y.; Cenci, A.; Hyma, K.E.; van den Houwe, I.; Hribova, E.; Courtois, B.; Roux, N. A Genome-Wide Association Study on the Seedless Phenotype in Banana (Musa spp.) Reveals the Potential of a Selected Panel to Detect Candidate Genes in a Vegetatively Propagated Crop. PLoS ONE 2016, 11, e0154448. [Google Scholar] [CrossRef] [Green Version]
- Boyaci, H.F.; Oguz, A.; Yazici, K.M.; Eren, A. The Efficacy of Endogenous Gibberellic Acid for Parthenocarpy in Eggplant (Solanum Melongena L.). Afr. J. Biotechnol. 2013, 10, 7. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Q.; Albrecht, U.; Shatters, R.G.; Stange, R.; McCollum, G.; Zhang, S.; Fan, C.; Stover, E. Comparative transcriptome analysis during early fruit development between three seedy citrus genotypes and their seedless mutants. Hortic. Res. 2017, 4, 17041. [Google Scholar] [CrossRef]
- Mesejo, C.; Yuste, R.; Martínez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (Citrus clementina). Physiol. Plant. 2013, 148, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Carbonell-Bejerano, P.; Torres-Pérez, R.; Nebish, A.; Martínez, Ó.; Rey, M.; Aroutiounian, R.; Ibáñez, J.; Martínez-Zapater, J.M. Developmental, transcriptome, and genetic alterations associated with parthenocarpy in the grapevine seedless somatic variant Corinto bianco. J. Exp. Bot. 2016, 67, 259–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledbetter, C.A.; Ramming, D.W. Seedlessness in Grapes. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1989; Volume 11, pp. 159–184. ISBN 978-1-118-06084-1. [Google Scholar]
- Honda, I.; Matsunaga, H.; Kikuchi, K.; Matsuo, S.; Fukuda, M. Identification of pepper (Capsicum annuum L.) accessions with large or small fruit that have a high degree of parthenocarpy. Sci. Hortic. 2012, 135, 68–70. [Google Scholar] [CrossRef]
- Nogueira, D.W.; Maluf, W.R.; dos Reis Figueira, A.; Maciel, G.M.; Gomes, L.A.A.; Benavente, C.A.T. Combining ability of summer-squash lines with different degrees of parthenocarpy and PRSV-W resistance. Genet. Mol. Biol. 2011, 34, 616–623. [Google Scholar] [CrossRef]
- Che, G.; Zhang, X. Molecular basis of cucumber fruit domestication. Curr. Opin. Plant Biol. 2019, 47, 38–46. [Google Scholar] [CrossRef]




| Species | Germplasm Name or Mutation Description | Source of Parthenocarpy |
|---|---|---|
| Apple | Spencer Seedless, Wellington Bloomless, Rae Ime [216] Wickson [217] | Natural |
| Banana | All consumed bananas are parthenocarpic. Examples of variety names: Blue Java [218] and Cavendish [204] | Natural (obligatory) |
| Cucumber | Camaro, Kalunga, Katrina, Socrates, Manny, Manar, Jawell, Picolino, Taurus, Tasty Jade, Tasty Green, Corinto, Lisboa, Alcazar, Sweet Success [213] | Natural |
| Eggplant | Talina, Galine [219] | Natural |
| Satsuma mandarin | Seedless Fallglo [220] | Induced mutation |
| Clementine | Marisol, Clemenules [221] | Natural |
| Grape | Corinto bianco [222], Black Corinth [223] | Natural |
| Grapefruit | Henderson, Marsh, Redblush [219] | Induced mutations |
| Pepper | Shishitoh [224] | Natural |
| Summer squash | Whitaker [225] | Natural |
| Sweet orange | US Seedless Pineapple [220] | Induced mutation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maupilé, L.; Boualem, A.; Chaïb, J.; Bendahmane, A. A Flashforward Look into Solutions for Fruit and Vegetable Production. Genes 2022, 13, 1886. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13101886
Maupilé L, Boualem A, Chaïb J, Bendahmane A. A Flashforward Look into Solutions for Fruit and Vegetable Production. Genes. 2022; 13(10):1886. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13101886
Chicago/Turabian StyleMaupilé, Léa, Adnane Boualem, Jamila Chaïb, and Abdelhafid Bendahmane. 2022. "A Flashforward Look into Solutions for Fruit and Vegetable Production" Genes 13, no. 10: 1886. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13101886