Insights of Improved Aroma under Additional Nitrogen Application at Booting Stage in Fragrant Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
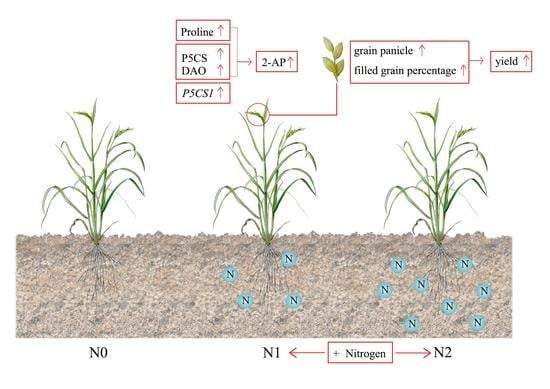
2.2. Treatments and Plant Sampling
2.3. Badh2 Sequences
2.4. Determination of Grain 2AP, Proline, P5C, Δ1-Pyrroline and GABA Contents
2.5. Determination of Enzymes Involved in 2-AP Biosynthesis in Grains
2.6. Real-Time Quantitative RT-PCR
2.7. Determination of Yield and Yield-Related Traits
2.8. Statistical Analyses
3. Results
3.1. Comparison of BADH2 Gene Sequence in Fragrant Rice
3.2. N-Induced Regulations in 2-AP, P5C, Δ1-Pyrroline, GABA, Proline and Methylglyoxal Levels in Grains
3.3. Effects of N on Genes Involved in 2AP Biosynthesis in Rice
3.4. N Application Modulates the Enzyme Involved in 2-AP Biosynthesis
3.5. Relationships between 2AP, Enzymes and Mid-Products of 2AP Biosynthesis
3.6. Effect of N on Yield and Yield-Related Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, L.; Ashraf, U.; Cheng, S.; Rao, G.; Mo, Z.; Tian, H.; Pan, S.; Tang, X. Short-term water management at early filling stage improves early-season rice performance under high temperature stress in South China. Eur. J. Agron. 2017, 90, 117–126. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Ashraf, U.; Wang, C.; He, L.; Wei, X.; Zheng, A.; Mo, Z.; Tang, X. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Buttery, R.G.; Ling, L.C. 2-acetyl-1-pyrroline an important aroma component of cooked rice. Chem. Ind. 1982, 23, 958–959. [Google Scholar]
- Lorieux, M.; Petrov, M.; Huang, N.; Guiderdoni, E.; Ghesquière, A. Aroma in rice: Genetic analysis of a quantitative trait. Theor. Appl. Genet. 1996, 93, 1145–1151. [Google Scholar] [CrossRef]
- Mo, Z.; Lei, S.; Ashraf, U.; Khan, I.; Li, Y.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Silicon fertilization modulates 2-acetyl-1-pyrroline content, yield formation and grain quality of aromatic rice. J. Cereal Sci. 2017, 75, 17–24. [Google Scholar] [CrossRef]
- Wongpornchai, S. Effects of drying methods and storage time on the aroma and milling quality of rice (Oryza sativa L.) cv. Khao Dawk Mali 105. Food Chem. 2004, 87, 407–414. [Google Scholar] [CrossRef]
- Mo, Z.; Li, Y.; Nie, J.; He, L.; Pan, S.; Duan, M.; Tian, H.; Xiao, L.; Zhong, K.; Tang, X. Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl-1-pyrroline (2AP) formation in fragrant rice. Rice 2019, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, L.M.T.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.E.; Henry, R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yang, Y.; Shi, W.; Ji, Q.; He, F.; Zhang, Z.; Cheng, Z.; Liu, X.; Xu, M. Badh2, Encoding Betaine Aldehyde Dehydrogenase, Inhibits the Biosynthesis of 2-Acetyl-1-Pyrroline, a Major Component in Rice Fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Kaikavoosi, K.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. 2-Acetyl-1-Pyrroline Augmentation in Scented indica Rice (Oryza sativa L.) Varieties Through Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) Gene Transformation. Appl. Biochem. Biotechnol. 2015, 177, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Singh, U. Integrated nitrogen fertilization for intensive and sustainable agriculture. J. Crop Improv. 2005, 15, 213–257. [Google Scholar] [CrossRef]
- Djaman, K.; Higgins, C.; O’Neill, M.; Begay, S.; Koudahe, K.; Allen, S. Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico. Insects 2019, 10, 369. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, S.; Kasahara, K.; Matsui, S.; Tokuda, M.; Saikawa, Y. Orange Leafhopper Cicadulina bipunctata Feeding Induces Gall Formation Nitrogen Dependently and Regulates Gibberellin Signaling. Plants 2020, 9, 1270. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Pan, S.; Yang, X. Effect of water-nitrogen interaction at tillering stage on aroma, grain yield and quality of aromatic rice. Acta Agric. Boreali-Sin. 2014, 29, 159–164. (In Chinese) [Google Scholar]
- Ren, Y.; Ashraf, U.; He, L.X.; Mo, Z.W.; Wang, F.; Wan, X.C.; Kong, H.; Ran, X.L.; Tang, X.R. Irrigation and nitrogen management practices affect grain yield and 2-acetyl-1-pyrroline content in aromatic rice. Appl. Ecol. Environ. Res. 2017, 15, 1447–1460. [Google Scholar] [CrossRef]
- Deng, Q.Q.; Ashraf, U.; Cheng, S.R.; Sabir, S.R.; Mo, Z.W.; Pan, S.G.; Tian, H.; Duan, M.Y.; Tang, X.R. Mild drought in interaction with additional nitrogen dose at grain filling stage modulates 2-acetyl-1-pyrroline biosynthesis and grain yield in fragrant rice. Appl. Ecol. Environ. Res. 2018, 16, 7741–7758. [Google Scholar] [CrossRef]
- Zhong, Q.; Tang, X. Effects of nitrogen application on aroma of aromatic rice and their mechanism. Guangdong Agric. Sci. 2014, 41, 85–87. (In Chinese) [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mezl, V.A.; Knox, W.E. Properties and analysis of a stable derivative of pyrroline-5-carboxylic acid for use in metabolic studies. Anal. Biochem. 1976, 74, 430–440. [Google Scholar] [CrossRef]
- Holmstedt, B.; Larsson, L.; Tham, R. Further studies of a spectrophotometric method for the determination of diamine oxidase activity. Biochim. Biophys. Acta 1961, 48, 182–186. [Google Scholar] [CrossRef]
- Zhao, D.; Pu, X.; Zeng, Y.; Li, B.; Du, J.; Yang, S. Determination of the γ-aminobutyric acid in barley. J. Triticeae Crops China 2009, 1, 69–72. [Google Scholar]
- Yao, S.; Yang, T.; Zhao, L.; Xiong, S. The variation of γ-aminobutyric acid content in germinated brown rice among different cultivars. Sci. Agric. Sin. China 2008, 12, 3974–3982. [Google Scholar]
- Zhang, C.-S.; Lu, Q.; Verma, D.P.S. Removal of Feedback Inhibition of Δ1-Pyrroline-5-carboxylate Synthetase, a Bifunctional Enzyme Catalyzing the First Two Steps of Proline Biosynthesis in Plants. J. Biol. Chem. 1995, 270, 20491–20496. [Google Scholar] [CrossRef]
- Su, G.; An, Z.; Zhang, W.; Liu, Y. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J. Plant Physiol. 2005, 162, 1297–1303. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. Betaine aldehyde dehydrogenase in plants. Plant Biol. 2009, 11, 119–130. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Waters, D.L.E.; Brooks, L.O.; Henry, R.J. Fragrance in rice (Oryza sativa) is associated with reduced yield under salt treatment. Environ. Exp. Bot. 2010, 68, 292–300. [Google Scholar] [CrossRef]
- Ghosh, P.; Roychoudhury, A. Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: Comparison with non-aromatic varieties. 3 Biotech 2018, 8, 25. [Google Scholar] [CrossRef]
- Poonlaphdecha, J.; Maraval, I.; Roques, S.; Audebert, A.; Boulanger, R.; Bry, X.; Gunata, Z. Effect of Timing and Duration of Salt Treatment during Growth of a Fragrant Rice Variety on Yield and 2-Acetyl-1-pyrroline, Proline, and GABA Levels. J. Agric. Food Chem. 2012, 60, 3824–3830. [Google Scholar] [CrossRef]
- Sakthivel, K.; Sundaram, R.; Rani, N.S.; Balachandran, S.; Neeraja, C. Genetic and molecular basis of fragrance in rice. Biotechnol. Adv. 2009, 27, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Jian, S.-F.; Huang, J.; Jin, Q.-Y.; Cao, X.-C. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 135, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hinge, V.; Patil, H.; Nadaf, A. Comparative Characterization of Aroma Volatiles and Related Gene Expression Analysis at Vegetative and Mature Stages in Basmati and Non-Basmati Rice (Oryza sativa L.) Cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-C.; Teng, C.-S.; Chang, J.-L.; Chuang, H.-S.; Ho, C.-T.; Wu, M.-L. Biosynthetic Mechanism of 2-Acetyl-1-pyrroline and Its Relationship with Δ1-Pyrroline-5-carboxylic Acid and Methylglyoxal in Aromatic Rice (Oryza sativa L.) Callus. J. Agric. Food Chem. 2008, 56, 7399–7404. [Google Scholar] [CrossRef]
- Christophersen, C.; Struve, C. Structural Equilibrium and Ring-Chain Tautomerism of Aqueous Solutions of 4-Aminobutyraldehyde. Heterocycles 2003, 60, 1907–1914. [Google Scholar] [CrossRef]
- He, Q.; Yu, J.; Kim, T.-S.; Cho, Y.-H.; Lee, Y.-S.; Park, Y.-J. Resequencing Reveals Different Domestication Rate for BADH1 and BADH2 in Rice (Oryza sativa). PLoS ONE 2015, 10, e0134801. [Google Scholar] [CrossRef]
- Niu, X.; Tang, W.; Huang, W.; Ren, G.; Wang, Q.; Luo, D.; Xiao, Y.; Yang, S.; Wang, F.; Lu, B.-R.; et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.). BMC Plant Biol. 2008, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Prodhan, Z.; Faruq, G.; Taha, R.M.; Rashid, K.A. Agronomic, Transcriptomic and Metabolomic Expression Analysis of Aroma Gene (badh2) under Different Temperature Regimes in Rice. Int. J. Agric. Biol. 2017, 19, 569–576. [Google Scholar] [CrossRef]





| Gene Name | Accession No. | Primer Sequences |
|---|---|---|
| OsBADH2-E7 | AB096083 | F: 5′-CTATCCTCTCCTGATGGCAAC-3′ |
| R: 5′-TCATGCAAGCCGATCAACC-3′ | ||
| P5CS1 | AK101230 | F: 5′-GAGGTTGGCATAAGCACAG-3′ |
| R: 5′-CTCCCTTGTCGCCGTTC-3′ | ||
| DAO | AK099435 | F: 5′-TGGCAAGATAGAAGCAGAAGT-3′ |
| R: 5′-GTCCATACGGGCAACAAA-3′ |
| Index | 2-AP | P5C | Δ1-pyroline | Proline | GABA | PRODH | P5CS | DAO | BADH |
|---|---|---|---|---|---|---|---|---|---|
| 2-AP | 1 | −0.237 | −0.561 | 0.346 | 0.146 | −0.016 | 0.236 | 0.836 * | 0.186 |
| P5C | - | 1 | 0.483 | 0.554 | −0.735 | −0.757 | 0.537 | −0.011 | −0.786 |
| Δ1-pyroline | - | - | 1 | 0.179 | −0.448 | −0.408 | 0.441 | −0.489 | −0.481 |
| proline | - | - | - | 1 | −0.049 | −0.687 | 0.803 | 0.651 | −0.773 |
| GABA | - | - | - | - | 1 | 0.595 | −0.362 | 0.174 | 0.388 |
| PRODH | - | - | - | - | - | 1 | −0.902 * | −0.394 | 0.922 ** |
| P5CS | - | - | - | - | - | - | 1 | 0.542 | −0.830 * |
| DAO | - | - | - | - | - | - | - | 1 | −0.277 |
| BADH | - | - | - | - | - | - | - | - | 1 |
| Cultivar | Treatment | Panicle Number Pot−1 | Grain Panicle−1 | Filled Grain Percentage (%) | 1000 Grain Weight (g) | Yield (t/hm2) |
|---|---|---|---|---|---|---|
| N0 | 34.23 a | 85.22 b | 66.51 b | 22.76 a | 4.41 b | |
| Meixiangzhan2 | N1 | 32.56 a | 95.44 a | 70.52 a | 21.9 a | 4.79 ab |
| N2 | 34.85 a | 99.62 a | 79.89 a | 18.45 b | 5.11 a | |
| N0 | 29.66 a | 76.66 b | 63.87 a | 17.68 a | 2.81 b | |
| Xiangyaxiangzhan | N1 | 30.11 a | 75.75 b | 62.73 a | 17.69 a | 2.53 b |
| N2 | 29.88 a | 96.85 a | 64.11 a | 16.96 a | 3.15 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, G.; Huang, S.; Ashraf, U.; Qiao, J.; Zheng, A.; Zhou, Q.; Li, L.; Wan, X. Insights of Improved Aroma under Additional Nitrogen Application at Booting Stage in Fragrant Rice. Genes 2022, 13, 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13112092
Bao G, Huang S, Ashraf U, Qiao J, Zheng A, Zhou Q, Li L, Wan X. Insights of Improved Aroma under Additional Nitrogen Application at Booting Stage in Fragrant Rice. Genes. 2022; 13(11):2092. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13112092
Chicago/Turabian StyleBao, Gegen, Suihua Huang, Umair Ashraf, Jingxuan Qiao, Axiang Zheng, Qi Zhou, Lin Li, and Xiaorong Wan. 2022. "Insights of Improved Aroma under Additional Nitrogen Application at Booting Stage in Fragrant Rice" Genes 13, no. 11: 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13112092