UNC5C: Novel Gene Associated with Psychiatric Disorders Impacts Dysregulation of Axon Guidance Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Library Preparation and NGS Analysis
2.2. Data Analysis
3. Results
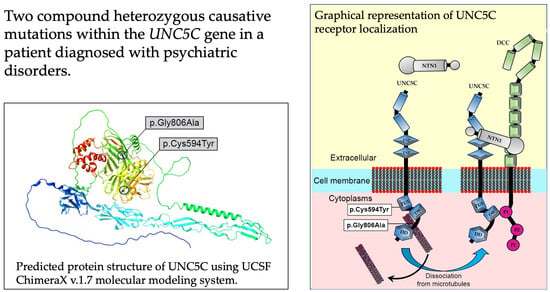
3.1. Clinical Report
3.2. Next-Generation Sequencing
3.3. In Silico Prediction
4. Discussion
4.1. UNC5C Gene Variant Identification
4.2. Impact of Observed Variants on Axon Guidance Signaling
4.3. Impact of Observed Variants on Functional Cytoplasmic Domains
4.4. Further Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surís, A.; Holliday, R.; North, C. The Evolution of the Classification of Psychiatric Disorders. Behav. Sci. 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.; Morgan, T.A.; Stanton, K. The Severity of Psychiatric Disorders. World Psychiatry 2018, 17, 258–275. [Google Scholar] [CrossRef]
- Hombali, A.; Seow, E.; Yuan, Q.; Chang, S.H.S.; Satghare, P.; Kumar, S.; Verma, S.K.; Mok, Y.M.; Chong, S.A.; Subramaniam, M. Prevalence and Correlates of Sleep Disorder Symptoms in Psychiatric Disorders. Psychiatry Res. 2019, 279, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Copeland, W.E.; Alaie, I.; Jonsson, U.; Shanahan, L. Associations of Childhood and Adolescent Depression With Adult Psychiatric and Functional Outcomes. J. Am. Acad. Child. Adolesc. Psychiatry 2021, 60, 604–611. [Google Scholar] [CrossRef]
- Keefe, J.R.; Kim, T.T.; DeRubeis, R.J.; Streiner, D.L.; Links, P.S.; McMain, S.F. Treatment Selection in Borderline Personality Disorder between Dialectical Behavior Therapy and Psychodynamic Psychiatric Management. Psychol. Med. 2021, 51, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sacristán, A.; Grosdidier, S.; Valverde, O.; Torrens, M.; Bravo, À.; Piñero, J.; Sanz, F.; Furlong, L.I. PsyGeNET: A Knowledge Platform on Psychiatric Disorders and Their Genes. Bioinformatics 2015, 31, 3075–3077. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Lyall, L.M.; Bethlehem, R.A.I.; Ferguson, A.; Strawbridge, R.J.; Lyall, D.M.; Cullen, B.; Graham, N.; Johnston, K.J.A.; Bailey, M.E.S.; et al. Novel Genome-Wide Associations for Anhedonia, Genetic Correlation with Psychiatric Disorders, and Polygenic Association with Brain Structure. Transl. Psychiatry 2019, 9, 327. [Google Scholar] [CrossRef]
- Baselmans, B.M.L.; Yengo, L.; van Rheenen, W.; Wray, N.R. Risk in Relatives, Heritability, SNP-Based Heritability, and Genetic Correlations in Psychiatric Disorders: A Review. Biol. Psychiatry 2021, 89, 11–19. [Google Scholar] [CrossRef]
- Low, D.M.; Bentley, K.H.; Ghosh, S.S. Automated Assessment of Psychiatric Disorders Using Speech: A Systematic Review. Laryngoscope Investig. Otolaryngol. 2020, 5, 96–116. [Google Scholar] [CrossRef]
- Brennand, K.J.; Simone, A.; Tran, N.; Gage, F.H. Modeling Psychiatric Disorders at the Cellular and Network Levels. Mol. Psychiatry 2012, 17, 1239–1253. [Google Scholar] [CrossRef]
- Kofink, D.; Boks, M.P.M.; Timmers, H.T.M.; Kas, M.J. Epigenetic Dynamics in Psychiatric Disorders: Environmental Programming of Neurodevelopmental Processes. Neurosci. Biobehav. Rev. 2013, 37, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.H.J. Toward Developmental Models of Psychiatric Disorders in Zebrafish. Front. Neural Circuits 2013, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Druart, M.; Le Magueresse, C. Emerging Roles of Complement in Psychiatric Disorders. Front. Psychiatry 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Copeland, W.E.; Shanahan, L.; Costello, E.J.; Angold, A. Childhood and Adolescent Psychiatric Disorders as Predictors of Young Adult Disorders. Arch. Gen. Psychiatry 2009, 66, 764. [Google Scholar] [CrossRef] [PubMed]
- Van Battum, E.Y.; Brignani, S.; Pasterkamp, R.J. Axon Guidance Proteins in Neurological Disorders. Lancet Neurol. 2015, 14, 532–546. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Wu, T.; Zhu, S.; Deng, L.; Cui, G. Axon Guidance Pathway Genes Are Associated with Schizophrenia Risk. Exp. Ther. Med. 2018, 16, 4519–4526. [Google Scholar] [CrossRef]
- Gong, Q.; Scarpazza, C.; Dai, J.; He, M.; Xu, X.; Shi, Y.; Zhou, B.; Vieira, S.; McCrory, E.; Ai, Y.; et al. A Transdiagnostic Neuroanatomical Signature of Psychiatric Illness. Neuropsychopharmacology 2019, 44, 869–875. [Google Scholar] [CrossRef]
- Grant, A.; Fathalli, F.; Rouleau, G.; Joober, R.; Flores, C. Association between Schizophrenia and Genetic Variation in DCC: A Case–Control Study. Schizophr. Res. 2012, 137, 26–31. [Google Scholar] [CrossRef]
- Bouilly, J.; Messina, A.; Papadakis, G.; Cassatella, D.; Xu, C.; Acierno, J.S.; Tata, B.; Sykiotis, G.; Santini, S.; Sidis, Y.; et al. DCC/NTN1 Complex Mutations in Patients with Congenital Hypogonadotropic Hypogonadism Impair GnRH Neuron Development. Hum. Mol. Genet. 2018, 27, 359–372. [Google Scholar] [CrossRef]
- Vosberg, D.E.; Leyton, M.; Flores, C. The Netrin-1/DCC Guidance System: Dopamine Pathway Maturation and Psychiatric Disorders Emerging in Adolescence. Mol. Psychiatry 2020, 25, 297–307. [Google Scholar] [CrossRef]
- Williams, M.E.; Lu, X.; McKenna, W.L.; Washington, R.; Boyette, A.; Strickland, P.; Dillon, A.; Kaprielian, Z.; Tessier-Lavigne, M.; Hinck, L. UNC5A Promotes Neuronal Apoptosis during Spinal Cord Development Independent of Netrin-1. Nat. Neurosci. 2006, 9, 996–998. [Google Scholar] [CrossRef]
- Poon, V.Y.; Klassen, M.P.; Shen, K. UNC-6/Netrin and Its Receptor UNC-5 Locally Exclude Presynaptic Components from Dendrites. Nature 2008, 455, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Bando, Y.; Sato, K. Involvement of Netrins and Their Receptors in Neuronal Migration in the Cerebral Cortex. Front. Cell Dev. Biol. 2021, 8, 590009. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.K.; Jevince, A.R.; Hinck, L.; Ackerman, S.L.; Lu, X.; Tessier-Lavigne, M.; Kaprielian, Z. UNC5C Is Required for Spinal Accessory Motor Neuron Development. Mol. Cell. Neurosci. 2007, 35, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Manitt, C.; Labelle-Dumais, C.; Eng, C.; Grant, A.; Mimee, A.; Stroh, T.; Flores, C. Peri-Pubertal Emergence of UNC-5 Homologue Expression by Dopamine Neurons in Rodents. PLoS ONE 2010, 5, e11463. [Google Scholar] [CrossRef] [PubMed]
- Kaukas, L.; Holmes, J.L.; Rahimi, F.; Collins-Praino, L.; Corrigan, F. Injury during Adolescence Leads to Sex-Specific Executive Function Deficits in Adulthood in a Pre-Clinical Model of Mild Traumatic Brain Injury. Behav. Brain Res. 2021, 402, 113067. [Google Scholar] [CrossRef] [PubMed]
- Hoops, D.; Kyne, R.F.; Salameh, S.; MacGowan, D.; Avramescu, R.G.; Ewing, E.; He, A.T.; Orsini, T.; Durand, A.; Popescu, C.; et al. The Scheduling of Adolescence with Netrin-1 and UNC5C. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ahmed Zaky, E. Adolescence; a Crucial Transitional Stage in Human Life. J. Child. Adolesc. Behav. 2016, 4, 115–116. [Google Scholar] [CrossRef]
- Cataldo, I.; Lepri, B.; Neoh, M.J.Y.; Esposito, G. Social Media Usage and Development of Psychiatric Disorders in Childhood and Adolescence: A Review. Front. Psychiatry 2021, 11, 508595. [Google Scholar] [CrossRef]
- Paradisi, A.; Creveaux, M.; Gibert, B.; Devailly, G.; Redoulez, E.; Neves, D.; Cleyssac, E.; Treilleux, I.; Klein, C.; Niederfellner, G.; et al. Combining Chemotherapeutic Agents and Netrin-1 Interference Potentiates Cancer Cell Death. EMBO Mol. Med. 2013, 5, 1821–1834. [Google Scholar] [CrossRef]
- Bhat, S.A.; Gurtoo, S.; Deolankar, S.C.; Fazili, K.M.; Advani, J.; Shetty, R.; Prasad, T.S.K.; Andrabi, S.; Subbannayya, Y. A Network Map of Netrin Receptor UNC5B-Mediated Signaling. J. Cell Commun. Signal 2019, 13, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, Z.; Jin, H.; Wu, H.; Yu, C.; Wen, W.; Chan, L.-N.; Wen, Z.; Zhang, M. Autoinhibition of UNC5b Revealed by the Cytoplasmic Domain Structure of the Receptor. Mol. Cell 2009, 33, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Ipsaro, J.J.; Mondragón, A. Structurally Similar but Functionally Diverse ZU5 Domains in Human Erythrocyte Ankyrin. J. Mol. Biol. 2012, 417, 336–350. [Google Scholar] [CrossRef] [PubMed]
- King, J.M.; Tan, C.J.P.; Thomason, N.C.; White, A.R.; Shen, L.; Turner, J.R. Zonula Occludens-1 ZU5 Domain Contributes Essential Stabilizing Interactions at the Tight Junction. FASEB J. 2016, 30, 1250–1257. [Google Scholar] [CrossRef]
- Purohit, A.A.; Li, W.; Qu, C.; Dwyer, T.; Shao, Q.; Guan, K.-L.; Liu, G. Down Syndrome Cell Adhesion Molecule (DSCAM) Associates with Uncoordinated-5C (UNC5C) in Netrin-1-Mediated Growth Cone Collapse. J. Biol. Chem. 2012, 287, 27126–27138. [Google Scholar] [CrossRef]
- Goldschneider, D.; Mehlen, P. Dependence Receptors: A New Paradigm in Cell Signaling and Cancer Therapy. Oncogene 2010, 29, 1865–1882. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Nakagawara, A. UNC5 Dependence Receptor Family in Human Cancer: A Controllable Double-Edged Sword. Cancer Lett. 2021, 516, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Larrivée, B.; Eichmann, A. Netrins and UNC5 Receptors in Angiogenesis. Angiogenesis 2008, 11, 23–29. [Google Scholar] [CrossRef]
- Castets, M.; Mehlen, P. Netrin-1 Role in Angiogenesis: To Be or Not to Be a pro-Angiogenic Factor? Cell Cycle 2010, 9, 1466–1471. [Google Scholar] [CrossRef]
- Yuan, M.; Xie, F.; Xia, X.; Zhong, K.; Lian, L.; Zhang, S.; Yuan, L.; Ye, J. UNC5C-knockdown Enhances the Growth and Metastasis of Breast Cancer Cells by Potentiating the Integrin α6/Β4 Signaling Pathway. Int. J. Oncol. 2019, 56, 139–150. [Google Scholar] [CrossRef]
- Miller, A.H. Beyond Depression: The Expanding Role of Inflammation in Psychiatric Disorders. World Psychiatry 2020, 19, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, P.; Liu, S.; Jing, S.; Lin, J.; Yang, J.; Zhu, Y.; Yu, M. A Global Integrated Analysis of UNC5C Down-Regulation in Cancers: Insights from Mechanism and Combined Treatment Strategy. Biomed. Pharmacother. 2021, 138, 111355. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Yang, T.; Huang, H.; Alarmanazi, F.; Liu, G. Uncoupling of UNC5C with Polymerized TUBB3 in Microtubules Mediates Netrin-1 Repulsion. J. Neurosci. 2017, 37, 5620–5633. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jang, S.-W.; Okada, M.; Chan, C.-B.; Feng, Y.; Liu, Y.; Luo, S.-W.; Hong, Y.; Rama, N.; Xiong, W.-C.; et al. Netrin-1 Mediates Neuronal Survival through PIKE-L Interaction with the Dependence Receptor UNC5B. Nat. Cell Biol. 2008, 10, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Graham, R. F2–01–01: A Rare Variant Alters UNC5C Function and Predisposes to Alzheimer’s Disease. Alzheimer’s Dement. 2013, 9, 311. [Google Scholar] [CrossRef]
- Ackerman, S.L.; Knowles, B.B. Cloning and Mapping of TheUNC5CGene to Human Chromosome 4q21–Q23. Genomics 1998, 52, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ackerman, S.L. The UNC5C Netrin Receptor Regulates Dorsal Guidance of Mouse Hindbrain Axons. J. Neurosci. 2011, 31, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Peng, G.; Gao, J. Expression of Unc5 Family Genes in Zebrafish Brain during Embryonic Development. Gene Expr. Patterns 2013, 13, 311–318. [Google Scholar] [CrossRef]
- Chen, G.; Ahn, E.H.; Kang, S.S.; Xia, Y.; Liu, X.; Zhang, Z.; Ye, K. UNC5C Receptor Proteolytic Cleavage by Active AEP Promotes Dopaminergic Neuronal Degeneration in Parkinson’s Disease. Adv. Sci. 2022, 9, e2103396. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Bye, S.; Nurnberger, J.I.; Hodes, M.E.; Crisp, M. A Non-Organic and Non-Enzymatic Extraction Method Gives Higher Yields of Genomic DNA from Whole-Blood Samples than Do Nine Other Methods Tested. J. Biochem. Biophys. Methods 1992, 25, 193–205. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Frías-López, C.; Sánchez-Herrero, J.F.; Guirao-Rico, S.; Mora, E.; Arnedo, M.A.; Sánchez-Gracia, A.; Rozas, J. DOMINO: Development of Informative Molecular Markers for Phylogenetic and Genome-Wide Population Genetic Studies in Non-Model Organisms. Bioinformatics 2016, 32, 3753–3759. [Google Scholar] [CrossRef]
- Quinodoz, M.; Royer-Bertrand, B.; Cisarova, K.; Di Gioia, S.A.; Superti-Furga, A.; Rivolta, C. DOMINO: Using Machine Learning to Predict Genes Associated with Dominant Disorders. Am. J. Human. Genet. 2017, 101, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.S.; Liao, H.; Hong, C.; Chien, W.; Chen, C. Identification of Two Inherited Copy Number Variants in a Male with Autism Supports Two-hit and Compound Heterozygosity Models of Autism. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2012, 159B, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Canafoglia, L.; Gennaro, E.; Capovilla, G.; Gobbi, G.; Boni, A.; Beccaria, F.; Viri, M.; Michelucci, R.; Agazzi, P.; Assereto, S.; et al. Electroclinical Presentation and Genotype–Phenotype Relationships in Patients with Unverricht-Lundborg Disease Carrying Compound Heterozygous CSTB Point and Indel Mutations. Epilepsia 2012, 53, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Ruderfer, D.M.; Lim, E.T.; Genovese, G.; Moran, J.L.; Hultman, C.M.; Sullivan, P.F.; McCarroll, S.A.; Holmans, P.; Sklar, P.; Purcell, S.M. No Evidence for Rare Recessive and Compound Heterozygous Disruptive Variants in Schizophrenia. Eur. J. Human. Genet. 2015, 23, 555–557. [Google Scholar] [CrossRef]
- Rees, E.; Kirov, G.; Walters, J.T.; Richards, A.L.; Howrigan, D.; Kavanagh, D.H.; Pocklington, A.J.; Fromer, M.; Ruderfer, D.M.; Georgieva, L.; et al. Analysis of Exome Sequence in 604 Trios for Recessive Genotypes in Schizophrenia. Transl. Psychiatry 2015, 5, e607. [Google Scholar] [CrossRef]
- Alvarez-Mora, M.I.; Corominas, J.; Gilissen, C.; Sanchez, A.; Madrigal, I.; Rodriguez-Revenga, L. Novel Compound Heterozygous Mutation in TRAPPC9 Gene: The Relevance of Whole Genome Sequencing. Genes 2021, 12, 557. [Google Scholar] [CrossRef]
- Torres-Berrío, A.; Hernandez, G.; Nestler, E.J.; Flores, C. The Netrin-1/DCC Guidance Cue Pathway as a Molecular Target in Depression: Translational Evidence. Biol. Psychiatry 2020, 88, 611–624. [Google Scholar] [CrossRef]
- Li, H.-J.; Qu, N.; Hui, L.; Cai, X.; Zhang, C.-Y.; Zhong, B.-L.; Zhang, S.-F.; Chen, J.; Xia, B.; Wang, L.; et al. Further Confirmation of Netrin 1 Receptor (DCC) as a Depression Risk Gene via Integrations of Multi-Omics Data. Transl. Psychiatry 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Daubaras, M.; Dal Bo, G.; Flores, C. Target-Dependent Expression of the Netrin-1 Receptor, UNC5C, in Projection Neurons of the Ventral Tegmental Area. Neuroscience 2014, 260, 36–46. [Google Scholar] [CrossRef]
- Sánchez-Huertas, C.; Herrera, E. With the Permission of Microtubules: An Updated Overview on Microtubule Function During Axon Pathfinding. Front. Mol. Neurosci. 2021, 14, 759404. [Google Scholar] [CrossRef]
- Auger, M.L.; Schmidt, E.R.E.; Manitt, C.; Dal-Bo, G.; Pasterkamp, R.J.; Flores, C. Unc5c Haploinsufficient Phenotype: Striking Similarities with the Dcc Haploinsufficiency Model. Eur. J. Neurosci. 2013, 38, 2853–2863. [Google Scholar] [CrossRef]
- Chen, G.; Kang, S.S.; Wang, Z.; Ahn, E.H.; Xia, Y.; Liu, X.; Sandoval, I.M.; Manfredsson, F.P.; Zhang, Z.; Ye, K. Netrin-1 Receptor UNC5C Cleavage by Active δ-Secretase Enhances Neurodegeneration, Promoting Alzheimer’s Disease Pathologies. Sci. Adv. 2021, 7, eabe4499. [Google Scholar] [CrossRef] [PubMed]




| c.1781G>A (p.Cys594Tyr) | c.2417G>C (p.Gly806Ala) | |||
|---|---|---|---|---|
| In silico Predictive tool | Prediction | Score | Prediction | Score |
| BayesDeladdAF | Pathogenic Moderate | 0.2533 | Pathogenic Moderate | 0.2297 |
| MetaRNN | Pathogenic Moderate | 0.9091 | Pathogenic Supporting | 0.763 |
| MetaLR | Benign Supporting | 0.3355 | Benign Supporting | 0.3358 |
| MetaSVM | Benign Supporting | −0.4082 | Benign Supporting | −0.4582 |
| BayesDelnoAF | Uncertain | 0.1261 | Uncertain | 0.09217 |
| REVEL | Uncertain | 0.648 | Uncertain | 0.486 |
| EIGEN | Pathogenic Supporting | 0.8281 | Pathogenic Moderate | 0.8924 |
| EIGEN PC | Pathogenic Supporting | 0.7964 | Pathogenic Moderate | 0.8802 |
| M-CAP | Benign Supporting | 0.05996 | Benign Moderate | 0.01793 |
| SIFT | Tolerated | 0.095 | Tolerated | 0.109 |
| FATHMM-MKL | Pathogenic Supporting | 0.9848 | Pathogenic Supporting | 0.9882 |
| FATHMM-XF | Pathogenic Moderate | 0.9595 | Pathogenic Supporting | 0.927 |
| LIST-S2 | Uncertain | 0.9707 | Pathogenic Supporting | 0.9807 |
| LRT | Pathogenic Supporting | 0 | Pathogenic Supporting | 0 |
| DEOGEN2 | Benign Supporting | 0.05267 | Benign Supporting | 0.3835 |
| FATHMM | Benign Supporting | 0.77 | Benign Supporting | 0.7 |
| BLOSUM | Uncertain | −6 | Uncertain | −1 |
| CADD | Uncertain | 243.999 | Uncertain | 253.999 |
| DANN | Uncertain | 0.9969 | Uncertain | 0.9987 |
| Mutation assessor | Uncertain | 2.2 | Uncertain | 2.71 |
| MutationTaster | Uncertain | 1 | Uncertain | 1 |
| MutPred | Pathogenic Moderate | 0.813 | Uncertain | 0.537 |
| MVP | Uncertain | 0.8722 | Uncertain | 0.867 |
| PrimateAI | Pathogenic Supporting | 0.8076 | Uncertain | 0.7238 |
| PROVEAN | Deleterious | −7.48 | Deleterious | −4.16 |
| SIFT4G | Benign Supporting | 0.115 | Uncertain | 0.046 |
| Polyphen2 | Probably damaging | 0.993 | Probably damaging | 0.999 |
| GnomADEuropean Allele Frequency | Not found | Not found | ||
| Protein | LogLR (a) | Threpp Score (b) |
|---|---|---|
| UNC5C wild type | 1.365 | 13.803 |
| UNC5C p.C594Y | 1.303 | 13.803 |
| UNC5C p.G806A | 1.299 | 13.803 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treccarichi, S.; Failla, P.; Vinci, M.; Musumeci, A.; Gloria, A.; Vasta, A.; Calabrese, G.; Papa, C.; Federico, C.; Saccone, S.; et al. UNC5C: Novel Gene Associated with Psychiatric Disorders Impacts Dysregulation of Axon Guidance Pathways. Genes 2024, 15, 306. https://0-doi-org.brum.beds.ac.uk/10.3390/genes15030306
Treccarichi S, Failla P, Vinci M, Musumeci A, Gloria A, Vasta A, Calabrese G, Papa C, Federico C, Saccone S, et al. UNC5C: Novel Gene Associated with Psychiatric Disorders Impacts Dysregulation of Axon Guidance Pathways. Genes. 2024; 15(3):306. https://0-doi-org.brum.beds.ac.uk/10.3390/genes15030306
Chicago/Turabian StyleTreccarichi, Simone, Pinella Failla, Mirella Vinci, Antonino Musumeci, Angelo Gloria, Anna Vasta, Giuseppe Calabrese, Carla Papa, Concetta Federico, Salvatore Saccone, and et al. 2024. "UNC5C: Novel Gene Associated with Psychiatric Disorders Impacts Dysregulation of Axon Guidance Pathways" Genes 15, no. 3: 306. https://0-doi-org.brum.beds.ac.uk/10.3390/genes15030306