Chiral Recognition of Azo-Schiff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Preparation of Complexes
2.3. Physical Measurements
2.4. X-Ray Crystallography
2.5. Computational Methods
3. Results and Discussion
3.1. Ligands Exhibiting Spontaneous Resolution
3.2. Complexes of Enantiomers and Diastremers
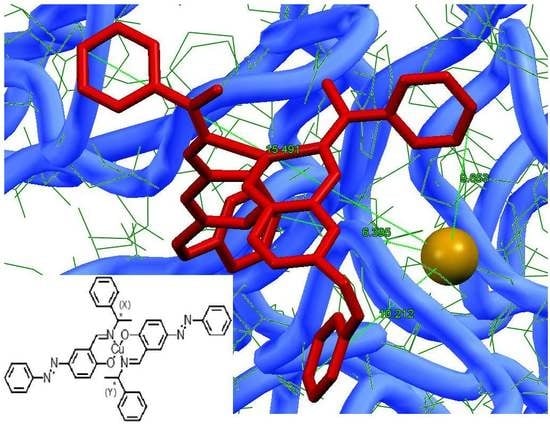
3.3. Hybrid Composites of Complexes in Laccase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mano, N.; de Poulpiquet, A. O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef] [PubMed]
- Goff, A.; Holzinger, M.; Cosnier, S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell. Mol. Life Sci. 2015, 72, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, L.; Stoj, C.; Taylor, A.B.; Hart, P.J.; Kosman, D.J.; Solomon, E.I. Shall We Dance? How A Multicopper Oxidase Chooses Its Electron Transfer Partner. Chem. Rev. 2007, 40, 445–452. [Google Scholar]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; MIta, H.; Sugiyama, T.; Tokita, Y.; Shirai, S.; Kano, K. Construction of a Multi-stacked Sheet-type Enzymatic Biofuel Cell. Electrochemistry 2014, 82, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Solomon, E.I.; Szilagyi, R.K.; DeBeer George, S.; Basumallick, L. Electronic structures of metal sites in proteins and models: Contributions to function in blue copper proteins. Chem. Rev. 2004, 104, 419–458. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, Y.; Tsuda, E.; Takase, M.; Yoshida, N.; Takeuchi, Y.; Mitsumoto, Y.; Akitsu, T. Spectroscopic and Electrochemical Studies on Metalloprotein (Laccase) and Cu(II) Complex Mediators As Model Systems for Biofuel Cell Cathodes. In Threonine: Food Sources, Functions and Health Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; Chapter 4; pp. 73–86. [Google Scholar]
- Takeuchi, Y.; Akitsu, T. Anthraquinone Derivative Chiral Schiff Base Copper(II) Complexes for Enzyme Type Bio-Fuel Cell Mediators. J. Electr. Eng. 2016, 4, 189–195. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Sunaga, N.; Akitsu, T. Anthraquinone and L-amino Acid Derivatives Schiff Base Cu(II) Complexes as a Mediator between Cathode of Biofuel Cell and Oxygen-reducing Laccase. Trend Green Chem. 2017, 3, 3. [Google Scholar] [CrossRef]
- Mitsumoto, Y.; Sunaga, N.; Akitsu, T. Polarized light induced molecular orientation in laccase for chiral azosalen Mn(II), Co(II), Ni(II), Cu(II), Zn(II) mediators toward application for biofuel cell. SciFed J. Chem. Res. 2017, 1, 1. [Google Scholar]
- Sano, A.; Yagi, S.; Haraguchi, T.; Akitsu, T. Synthesis of Mn (II) and, Cu (II) complexes including azobenzene and its application to mediators of laccase for biofuel cells. J. Indian Chem. Soc. 2018, 95, 487–494. [Google Scholar]
- Aritake, Y.; Watanabe, Y.; Akitsu, T. Chiral photochromic Schiff base: 4-phenylazo-2-[[(R)-(1-phenylethyl)imino]methyl]phenol. Acta Crystallogr. E 2010, 66, o749. [Google Scholar] [CrossRef] [PubMed]
- Kominato, C.; Akitsu, T. Photoinduced molecular orientation of catalytic-like chiral azo-Schiff base complexes in PMMA or laccase matrices. Lett. Appl. NanoBioSci. 2015, 2, 264–270. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- CCDC CSD. Available online: https://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/Gold/ (accessed on 24 April 2019).
- PDB. Available online: http://www.rcsb.org/ (accessed on 24 April 2019).
- Akitsu, T.; Komorita, S.; Tamura, H. Diastereomers of copper(II) complexes exhibiting difference in coordination geometry by R- or S-1-phenylethylamine lignads in the solid state and structural conversion of crystals into solutions. Inorg. Chim. Acta 2003, 348, 25–32. [Google Scholar] [CrossRef]
- Xia, Z.; Jing, X.; He, C.; Wang, X.; Duan, C. Coordinative Alignment of Chiral Molecules to Control over the Chirality Transfer in Spontaneous Resolution and Asymmetric Catalysis. Sci. Rep. 2017, 7, 15418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Akitsu, T. Photofunctional supramolecular solution systems of chiral Schiff base nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes. Polyhedron 2007, 26, 2527–2535. [Google Scholar] [CrossRef]
- Yamazaki, A.; Akitsu, T. Polarized spectroscopy and polarized UV light-induced molecular orientation of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes and azobenzene in a PMMA film. RSC Adv. 2012, 2, 2975–2980. [Google Scholar] [CrossRef]
- Ito, M.; Akitsu, T.; Palafox, M.A. Theoretical interpretation of polarized light-induced supramolecular orientation on the basis of normal mode analysis of azobenzene as hybrid materials in PMMA with chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes. J. Appl. Solut. Chem. Model. 2016, 5, 30–47. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunitake, F.; Kim, J.-Y.; Yagi, S.; Yamzaki, S.; Haraguchi, T.; Akitsu, T. Chiral Recognition of Azo-Schiff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators. Symmetry 2019, 11, 666. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11050666
Kunitake F, Kim J-Y, Yagi S, Yamzaki S, Haraguchi T, Akitsu T. Chiral Recognition of Azo-Schiff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators. Symmetry. 2019; 11(5):666. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11050666
Chicago/Turabian StyleKunitake, Fuki, Jong-Yeon Kim, Shiomi Yagi, Shu Yamzaki, Tomoyuki Haraguchi, and Takashiro Akitsu. 2019. "Chiral Recognition of Azo-Schiff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators" Symmetry 11, no. 5: 666. https://0-doi-org.brum.beds.ac.uk/10.3390/sym11050666