Sintering Optimisation and Recovery of Aluminum and Sodium from Greek Bauxite Residue
Abstract
:1. Introduction
Previous Studies in Soda and Lime-Soda Sintering Processes
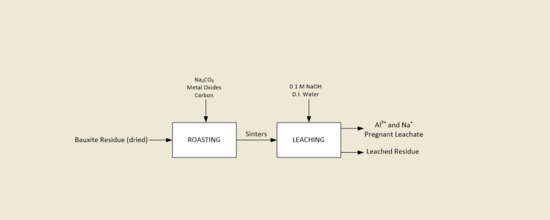
2. Materials and Methods
2.1. Characterisation of Bauxite Residue
2.2. Laboratorial Methods in Sintering Optimisation
3. Results and Discussion
3.1. Effect of Sintering Temperature and Retention Time on Metals Recoveries
3.2. Effect of Na2CO3 Excess on Metals Recoveries
3.3. Effect of Carbon Additions on Metals Recoveries
3.4. Effect of CaO Additions on Phase Transformations during Sintering Process
3.5. Effect of MgO Additions on Phase Transformation during Sintering Process
3.6. Effect of BaO Additions on Phase Transformations during Sintering Process
3.7. Experimental Results of Leaching Tests from 50% Excess Soda Sinters with CaO, MgO and BaO Additions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IAI. Current IAI Statistics: Alumina Production in Total for 2017. Available online: http://www.world-aluminum.org/statistics/alumina-production/ (accessed on 2 July 2018).
- Mytilineos, S.A. Metallurgy. Available online: http://www.mytilineos.gr/en-us/metallurgy-and-mining/activities (accessed on 3 July 2018).
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A Mineralogical Assessment on Residues after Acidic Leaching of Bauxite Residue (Red Mud) for Titanium Recovery. Metals 2017, 7, 458. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Major Metals from Bauxite Residue (Red Mud) by Alkali Roasting, Smelting, and Leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Comparative Analysis of Processes for Recovery of Rare Earths from Bauxite Residue. JOM 2016, 68, 2958–2962. [Google Scholar] [CrossRef]
- Avdibegovic, D.; Regadio, M.; Binnemans, K. Efficient separation of rare earths recovered by a supported ionic liquid from bauxite residue leachate. RSC Adv. 2018, 8, 11886–11893. [Google Scholar] [CrossRef] [Green Version]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 1–16. [Google Scholar] [CrossRef]
- Smith, P. Reactions of lime under high temperature Bayer digestion conditions. Hydrometallurgy 2017, 170, 16–23. [Google Scholar] [CrossRef]
- Mishra, B.; Gostu, S. Materials sustainability for environment: Red-mud treatment. Front. Chem. Sci. Eng. 2017, 11, 483–496. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Calle, C.M.; Davidson, R.H.; Glenn, A.M.; Jahanshahi, S.; Somerville, M.A.; Sparrow, G.J.; Zhang, L. Smelting of bauxite residue to form a soluble sodium aluminum silicate phase to recover alumina and soda. Trans. Inst. Min. Metall. Sect. C 2010, 119, 18–26. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Sofras, I.; Friedrich, B. Carbothermic reduction of red mud in an EAF and subsequent recovery of aluminum from the slag by pressure leaching in caustic solution. In Proceedings of the Bauxite Residue Valorisation and Best Practices (BR 2015), Leuven, Belgium, 5–7 October 2015; pp. 185–189. [Google Scholar]
- Kaußen, F.M.; Friedrich, B. Methods for Alkaline Recovery of Aluminum from Bauxite Residue. J. Sustain. Metall. 2016, 2, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Tam, P.W.Y.; Xakalashe, B.; Friedrich, B.; Panias, D.; Vassiliadou, V. Carbothermic Reduction of Bauxite Residue for Iron Recovery and Subsequent Aluminum Recovery from Slag Leaching. In Proceedings of the 35th International Conference and Exhibition of The International Committee for Study of Bauxite, Alumina & Aluminum (ICSOBA), Hamburg, Germany, 2–5 October 2017; pp. 603–613. [Google Scholar]
- Dobos, G.; Horváth, G.; Felföldi, Z.; Karšulin, M.C.E.; Marušić, R.E. Complex utilization of red mud including the production of pig iron, sodium hydroxide, alumina and building material. In Travaux ICSOBA; Hungary, International Colloquium on Alumina Production from Low Grade Bauxites: Žiar nad Hronom, Slovakia, 1974; pp. 151–158. [Google Scholar]
- Li, G.; Jiang, T.; Liu, M.; Zhou, T.; Fan, X.; Qiu, G. Beneficiation of High-Aluminum-Content Hematite Ore by Soda Ash Roasting. Min. Process. Extr Metall. Rev. 2010, 31, 150–164. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Kaußen, F.M.; Friedrich, B. Soda Sintering Process for the Mobilisation of Aluminum and Gallium in Red Mud. In Proceedings of the Bauxite Residue Valorisation and Best Practices Conference (BR 2015), Leuven, Belgium, 5–7 October 2015; pp. 157–164. [Google Scholar]
- Zheng, K.; Gerson, A.R.; Addai-Mensah, J.; Smart, R.S.C. The influence of sodium carbonate on sodium ahminosilicate crystallisation and solubility in sodium aluminate solutions. J. Cryst. Growth 1997, 171, 197–208. [Google Scholar] [CrossRef]
- Bánvölgyi, G. Scale Formation in Alumina Refineries. In Proceedings of the 34th International Conference and Exhibition of The International Committee for Study of Bauxite, Alumina & Aluminum (ICSOBA), St. Colomban, QC, Canada, 3–6 October 2016; pp. 101–114. [Google Scholar]
- Rivera, R.M.; Ounoughene, G.; Borra, C.R.; Binnemans, K.; Van Gerven, T. Neutralisation of bauxite residue by carbon dioxide prior to acidic leaching for metal recovery. Min. Eng. 2017, 112, 92–102. [Google Scholar] [CrossRef]
- Habashi, F. Karl Josef Bayer (1847–1904). In International Committee for Study of Bauxite, Alumina and Aluminum (ISCOBA), Movement of Scientists and the Production of Aluminum; ICSOBA Newsletter: St. Colomban, QC, Canada, 2015; pp. 9–13. [Google Scholar]
- Dennis, W. Metallurgy: 1863–1963; Aldine Transaction: Piscataway, NJ, USA, 2010. [Google Scholar]
- Alp, A.; Aydin, A.O. The Investigation of Efficient Conditions for Alumina Production from Diasporic Bauxites. Can. Metall. Q. 2002, 41, 41–46. [Google Scholar] [CrossRef]
- Alp, A.; Selim Goral, M. The effects of the additives, calcination and leach conditions for alumina production from red mud. Scand. J. Metall. 2003, 32, 301–305. [Google Scholar] [CrossRef]
- Panias, D.; Paspaliaris, I. Boehmite Process—A New Approach in Alumina Production. Erzmetall 2003, 56, 75–81. [Google Scholar]
- Wang, B.; Sun, H.-L.; Guo, D.; Zhang, X.-Z. Effect of Na2O on alumina leaching property and phase transformation of MgO-containing calcium aluminate slags. Trans. Nonferrous Metall. Soc. China 2011, 21, 2752–2757. [Google Scholar] [CrossRef]
- Kaußen, F.M.; Friedrich, B. Phase characterization and thermochemical simulation of (landfilled) bauxite residue (“red mud”) in different alkaline processes optimized for aluminum recovery. Hydromet 2018, 176, 49–61. [Google Scholar] [CrossRef]
- Smith, P. The processing of high silica bauxites–Review of existing and potential processes. Hydromet 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Meher, S.N.; Rout, A.K.; Padhi, B.K. Extraction of Alumina from Red Mud by Divalent Alkaline Earth Metal Soda Ash Sinter Process. Light Metals 2011, 2011, 231–236. [Google Scholar] [CrossRef]
- Meher, S.N.; Padhi, B.K. A novel method for extraction of alumina from red mud by divalent alkaline earth metal oxide and soda ash sinter process. Int. J. Environ. Waste Manag. 2014, 13, 231–245. [Google Scholar] [CrossRef]
- Raghavan, P.K.N.; Kshatriya, N.K.; Wawrynink, K. Recovery of Metal Values from Red Mud. In Light Metals 2011; Lindsay, S.J., Ed.; Springer International Publishing: Cham, Germany, 2016; pp. 103–106. [Google Scholar] [CrossRef]
- Hertel, T.; Blanpain, B.; Pontikes, Y. A Proposal for a 100% Use of Bauxite Residue Towards Inorganic Polymer Mortar. J. Sustain. Metall. 2016, 2, 394–404. [Google Scholar] [CrossRef]
- Tathavadkar, V.; Jha, A.; Fülöp, T.; Török, T.I.; Rédey, A. A Comparison of the Mineralogical Characteristics and Alkali Roasting Behaviour of Red Mud of Different Origins. In Proceedings of the Global Symposium on Recycling, Waste Treatment and Clean Technology (REWAS 2004), Madrid, Spain, 26–29 September 2004; pp. 401–410. [Google Scholar]
- Whittington, B.I. The chemistry of CaO and Ca(OH)2 relating to the Bayer process. Hydromet 1996, 43, 13–35. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyirő-Kósa, I.; Csákberényi-Malasics, D.; Tóth, Á.; Czitrovszky, A.; et al. The Red Mud Accident in Ajka (Hungary): Characterization and Potential Health Effects of Fugitive Dust. Env. Sci Technol 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Mohapatra, B.K.; Mishra, B.K.; Mishra, C.R. Investigation of Alumina Discharge into the Red Mud Pond at NALCO’s Alumina Refinery, Damanjodi, Orrisa, India. In Light Metals 2011; Lindsay, S.L., Ed.; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 2011; pp. 93–96. [Google Scholar]
- Meher, S.N.; Route, A.K.; Padhi, B.K. Recovery of Al and Na Values from Red Mud by BaO-Na2CO3 Sinter Process. J. Chem. 2011, 8, 1387–1393. [Google Scholar] [CrossRef]
- Meher, S.N.; Padhi, B.K. Effects of MgO and Na2CO3 Additives, Sintering Temperature and Leaching Conditions for Extraction of Alumina from Bayer’s Process Waste Residue (Red Mud). Chem. Sci. Trans. 2012, 1, 456–462. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Min. Eng 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Sajó, I.E. XDB Powder Diffraction Phase Analytical System, Version 3.0, User’s Guide; Aluterv-FKI: Budapest, Hungary, 2005. [Google Scholar]
- Hodge, H.; Tam, P.W.Y.; Vaughan, J.; Panias, D. Bauxite Residue Sinter Phase Transformations. In Proceedings of the 5th International Slag Valorisation Symposium, Leuven, Belgium, 3–5 April 2017. [Google Scholar]
- Meher, S.N. Alumina extraction from red mud by CaCO3 and Na2CO3 sinter process. Int. J. Chem. Stud. 2016, 4, 122–127. [Google Scholar]






















| Oxides | Major Metals | Others | LOI * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | SiO2 | TiO2 | Na2O | CaO | V2O5 | SO3 | ||
| wt % | 43.51 | 19.26 | 6.50 | 5.49 | 2.81 | 9.59 | 0.17 | 0.47 | 9.40 |
| Mineralogy | Anatase | Boehmite | Gibbsite | Hematite | Goethite | Perovskite | Cancrinite | Diaspore | Calcite | Rutile | Grossular | Chamosite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase percentage % | 1.0 | 2.1 | 1.0 | 37.5 | 5.2 | 5.2 | 11.5 | 13.0 | 4.2 | 0.5 | 14.6 | 2.1 |
| Mass Balance (kg) | Bauxite Residue | Na2CO3 | Sinter | CO2/CO | NaOH | Leached Residue | Metallurgical Coke |
|---|---|---|---|---|---|---|---|
| Soda only | 1 | 0.3 | 1.08 | 0.22 | 0.288 | 0.737 | - |
| Carbon + Soda | 1 | 0.3 | 0.905 | 0.318 | 0.288 | 0.6325 | 0.122 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, P.W.Y.; Panias, D.; Vassiliadou, V. Sintering Optimisation and Recovery of Aluminum and Sodium from Greek Bauxite Residue. Minerals 2019, 9, 571. https://0-doi-org.brum.beds.ac.uk/10.3390/min9100571
Tam PWY, Panias D, Vassiliadou V. Sintering Optimisation and Recovery of Aluminum and Sodium from Greek Bauxite Residue. Minerals. 2019; 9(10):571. https://0-doi-org.brum.beds.ac.uk/10.3390/min9100571
Chicago/Turabian StyleTam, Pritii Wai Yin, Dimitrios Panias, and Vicky Vassiliadou. 2019. "Sintering Optimisation and Recovery of Aluminum and Sodium from Greek Bauxite Residue" Minerals 9, no. 10: 571. https://0-doi-org.brum.beds.ac.uk/10.3390/min9100571