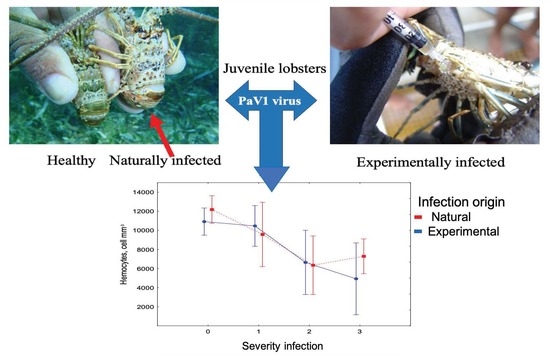
Immune Response to Natural and Experimental Infection of Panulirus argus Virus 1 (PaV1) in Juveniles of Caribbean Spiny Lobster
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Naturally Infected Juveniles
2.2. Preparation of the Viral Inoculum
2.3. Experimental Challenge
2.4. Sample Collection
2.5. Immunological Tests
2.6. Preparation of Plasma and Degranulated Hemocytes
2.7. Hemocyte Counts
2.8. Hemagglutination Activity
2.9. Total Phenoloxidase Activity (PO)
2.10. PCR
2.11. Histology
2.12. Determination of Molt Stage and Size
2.13. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. In Sustainability in Action; FAO: Rome, Italy, 2020; Volume 32, p. 244. [Google Scholar] [CrossRef]
- Small, H.J.; Pagenkopp, K.M. Reservoirs and alternate hosts for pathogens of commercially important crustaceans: A review. J. Invertebr. Pathol. 2011, 106, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Neil, D.M.; Peeler, E.J.; Shields, J.D.; Small, H.J.; Flegel, T.W.; Vlak, J.M.; Jones, B.; Morado, F.; Moss, S.; et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J. Invertebr. Pathol. 2012, 110, 141–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightner, D.V. Virus diseases of farmed shrimp in the Western Hemisphere (the Americas): A review. J. Invertebr. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef]
- Shields, J.D. The impact of pathogens on exploited populations of decapod crustaceans. J. Invertebr. Pathol. 2012, 110, 211–224. [Google Scholar] [CrossRef]
- Behringer, D.C. Diseases of wild and cultured juvenile crustaceans: Insights from below the minimum landing size. J. Invertebr. Pathol. 2012, 110, 225–233. [Google Scholar] [CrossRef]
- Ning, M.; Bi, J.; Sun, W.; Xie, X.; Huang, Y.; Gu, W.; Wang, W.; Qiao, Y.; Jiang, G.; Shen, H.; et al. Linolenic acid improves growth performance and immune status of Penaeus vannamei infected by enterocytozoon hepatopenaei. Aquaculture 2021, 535, 736397. [Google Scholar] [CrossRef]
- López, N.; Cuzon, G.; Gaxiola, G.; Taboada, G.; Valenzuela, M.; Pascual, C.; Sánchez, A.; Rosas, C. Physiological, nutritional and immunological role of dietary B 1-3 glucan and ascorbic acid 2-monophosphate in Litopenaeus vannamei juveniles. Aquaculture 2003, 224, 223–243. [Google Scholar] [CrossRef]
- Cock, J.; Gitterle, T.; Salazar, M.; Rye, M. Breeding for disease resistance of Penaeid shrimps. Aquaculture 2009, 286, 1–11. [Google Scholar] [CrossRef]
- Gitterle, T.; Ødegård, J.; Gjerde, B.; Rye, M.; Salte, R. Genetic parameters and accuracy of selection for resistance to White Spot Syndrome Virus (WSSV) in Penaeus (Litopenaeus) vannamei using different statistical models. Aquaculture 2006, 251, 210–218. [Google Scholar] [CrossRef]
- Baneth, G.; Harrus, S.; Ohnona, F.S.; Schlesinger, Y. Longitudinal quantification of Ehrlichia canis in experimental infection with comparison to natural infection. Vet. Microbiol. 2009, 136, 321–325. [Google Scholar] [CrossRef]
- Atkins, C.E.; Vaden, S.L.; Arther, R.G.; Ciszewski, D.K.; Davis, W.L.; Ensley, S.M.; Chopade, N.H. Renal effects of Dirofilaria immitis in experimentally and naturally infected cats. Vet. Parasitol. 2011, 176, 317–323. [Google Scholar] [CrossRef]
- Bonneaud, C.; Balenger, S.L.; Hill, G.E.; Russell, A.F. Experimental evidence for distinct costs of pathogenesis and immunity against a natural pathogen in a wild bird. Mol. Ecol. 2012, 21, 4787–4796. [Google Scholar] [CrossRef]
- Sorci, G. Immunity, resistance and tolerance in bird-parasite interactions. Parasite Immunol. 2013, 35, 350–361. [Google Scholar] [CrossRef]
- Moreno-García, M.; Condé, R.; Bello-Bedoy, R.; Lanz-Mendoza, H. The damage threshold hypothesis and the immune strategies of insects. Infect. Genet. Evol. 2014, 24, 25–33. [Google Scholar] [CrossRef]
- Subramaniam, K.; Behringer, D.C.; Bojko, J. A new family of DNA viruses causing disease in crustaceans from diverse aquatic biomes. mBio 2020, 11, e02938-19. [Google Scholar] [CrossRef] [Green Version]
- Shields, J.; Behringer, D. A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis. Aquat. Organ. 2004, 59, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Shields, J.; Small, H.; Reece, K.; Hartwig, C.; Cooper, R.; Ratzlaff, R. Detection of Panulirus argus Virus 1 (PaV1) in the Caribbean spiny lobster using fluorescence in situ hybridization (FISH). Dis. Aquat. Organ. 2006, 72, 185–192. [Google Scholar] [CrossRef]
- Lozano-Álvarez, E.; Briones-Fourzán, P.; Ramírez-Estévez, A.; Placencia-Sánchez, D.; Huchin-Mian, J.; Rodríguez-Canul, R. Prevalence of Panulirus argus Virus 1 (PaV1) and habitation patterns of healthy and diseased Caribbean spiny lobsters in shelter-limited habitats. Dis. Aquat. Organ. 2008, 80, 95–104. [Google Scholar] [CrossRef]
- Huchin-Mian, J.; Rodríguez-Canul, R.; Arias-Bañuelos, E.; Simá-Álvarez, R.; Pérez-Vega, J.; Briones-Fourzán, P.; Lozano-Álvarez, E. Presence of Panulirus argus Virus 1 (PaV1) in juvenile spiny lobsters Panulirus argus from the Caribbean coast of Mexico. Dis. Aquat. Organ. 2008, 79, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Huchin-Mian, J.; Briones-Fourzán, P.; Simá-Álvarez, V.; Cruz-Quintana, Y.; Pérez-Vega, J.; Lozano-Álvarez, E.; Pascual-Jiménez, C.; Rodríguez-Canul, R. Detection of Panulirus argus Virus 1 (PaV1) in exported frozen tails of subadult-adult Caribbean spiny lobsters Panulirus argus. Dis. Aquat. Organ. 2009, 86, 159–162. [Google Scholar] [CrossRef]
- Cruz Quintana, Y.; Rodríguez Canul, R.; Vidal Martínez, V. First evidence of Panulirus argus Virus 1 (PaV1) in spiny lobster from Cuba and clinical estimation of its prevalence. Dis. Aquat. Organ. 2011, 93, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.; Behringer, D.; Shields, J.; Baeza, A.; Aguilar-Perera, A.; Bush, P.; Dromer, C.; Herrera-Moreno, A.; Gittens, L.; Matthews, T.; et al. Distribution, prevalence, and genetic analysis of Panulirus argus virus 1 (PaV1) from the Caribbean Sea. Dis. Aquat. Organ. 2013, 104, 129–140. [Google Scholar] [CrossRef]
- Butler, M.; Behringer, D.; Shields, J. Transmission of Panulirus argus virus 1 (PaV1) and its effect on the survival of juvenile Caribbean spiny lobster. Dis. Aquat. Organ. 2008, 79, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Jiménez, C.; Huchin-Mian, J.P.; Simões, N.; Briones-Fourzán, P.; Lozano-Álvarez, E.; Sánchez-Arteaga, A.; Pérez-Vega, J.A.; Simá-Álvarez, R.; Rosas-Vazquez, C.; Rodríguez-Canul, R. Physiological and immunological characterization of Caribbean spiny lobsters Panulirus argus naturally infected with Panulirus argus Virus 1 (PaV1). Dis. Aquat. Organ. 2012, 100, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Pascual, C.; Zenteno, E.; Cuzon, G.; Suárez, J.; Sánchez, A.; Gaxiola, G.; Taboada, G.; Maldonado, T.; Rosas, C. Litopenaeus vannamei juveniles energetic balance and immunological response to dietary proteins. Aquaculture 2004, 239, 375–395. [Google Scholar] [CrossRef]
- Montgomery-Fullerton, M.; Cooper, R.; Kauffman, K.; Shields, J.; Ratzlaff, R. Detection of Panulirus argus Virus 1 in Caribbean spiny lobsters. Dis. Aquat. Organ. 2007, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, M.; Varghese, R.; Philip, R.; Mohandas, A.; Bright Singh, I.S. Pathological changes in Fenneropenaeus indicus experimentally infected with white spot virus and virus morphogenesis. J. Invertebr. Pathol. 2009, 102, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Álvarez, E. Pesquería, Dinámica Poblacional y Manejo de la Langosta Panulirus argus (Latreille, 1804) en la Bahía de la Ascensión, Q.R., México. Ph.D. Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 1992. [Google Scholar]
- Xu, P.; Janex, M.-L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Lyle, W.G.; MacDonald, C.D. Molt Stage Determination in the Hawaiian Spiny Lobster Panulirus marginatus. J. Crustac. Biol. 1983, 3, 208–216. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Gómez-Jiménez, S.; Portillo-Clark, G.; Vargas-Albores, F. In the spiny lobster (Panulirus interruptus) the prophenoloxidase is located in plasma not in haemocytes. Fish Shellfish Immunol. 2003, 14, 105–114. [Google Scholar] [CrossRef]
- Le Moullac, G.; Soyez, C.; Saulnier, D.; Ansquer, D.; Avarre, J.C.; Levy, P. Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol. 1998, 8, 621–629. [Google Scholar] [CrossRef]
- Ashida, M.; Söderhäll, K. The prophenoloxidase activating system in crayfish. Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 77, 21–26. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp. Biochem. Physiol.-C Pharmacol. Toxicol. Endocrinol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; World Aquaculture Society: Baton Rouge, LA, USA, 1988; Volume 2, 101p, Available online: https://cesarom.files.wordpress.com/2013/08/bell-lightner_1988_handbook-of-shrimp-histology.pdf (accessed on 5 May 2022).
- Zamora-Briseño, J.; Ruiz-May, E.; Elizalde-Contreras, J.M.; Hernández-Velázquez, I.; Hernández-Párez, A.; Fuentes-García, A.; Herrera-Salvatierra, N.; Briones-Fourzán, P.; Pascual-Jiménez, C.; Lozano-Álvarez, E.; et al. iTRAQ-Based proteomic profile analysis of the hepatopancreas of Caribbean spiny lobsters infected with Panulirus argus virus 1: Metabolic and physiological implications. Front. Microbiol. 2020, 11, 1084. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Borghetti, P.; Saleri, R.; Mocchegiani, E.; Corradi, A.; Martelli, P. Infection, immunity and the neuroendocrine response. Vet. Immunol. Immunopathol. 2009, 130, 141–162. [Google Scholar] [CrossRef]
- Söderhäll, K.; Häll, L. Lipopolysaccharide-induced activation of prophenoloxidase activating system in crayfish haemocyte lysate. BBA Gen. Subj. 1984, 797, 99–104. [Google Scholar] [CrossRef]
- Johansson, M.W.; Söderhäll, K. Isolation and purification of a cell adhesion factor from crayfish blood cells. J. Cell Biol. 1988, 106, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef]
- Aono, H.; Mori, K. Interaction between hemocytes and plasma is necessary for hemolymph coagulation in the spiny lobster, Panulirus japonicus. Comp. Biochem. Physiol. Part A Physiol. 1996, 113, 301–305. [Google Scholar] [CrossRef]
- Cheng, W.; Tsai, I.-H.; Huang, C.-J.; Chiang, P.-C.; Cheng, C.-H.; Yeh, M.-S. Cloning and characterization of hemolymph clottable proteins of kuruma prawn (Marsupenaeus japonicus) and white shrimp (Litopenaeus vannamei). Dev. Comp. Immunol. 2008, 32, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.G.; Omori, J.E.H.S.; Chong, C.; Hoodbhoy, T.; McKrell, N. Localization and roles of coagulogen and transglutaminase in hemolymph coagulation in decapod crustaceans. Comp. Biochem. Physiol. Part B Comp. Biochem. 1991, 100, 517–522. [Google Scholar] [CrossRef]
- Sung, H.H.; Chang, H.J.; Her, C.H.; Chang, J.C.; Song, Y.L. Phenoloxidase Activity of Hemocytes Derived from Penaeus monodon and Macrobrachium rosenbergii. J. Invertebr. Pathol. 1998, 71, 26–33. [Google Scholar] [CrossRef]
- Cerenius, L.; Kawabata, S.; Lee, B.L.; Nonaka, M.; Söderhäll, K. Proteolytic cascades and their involvement in invertebrate immunity. Trends. Biochem. Sci. 2010, 35, 575–583. [Google Scholar] [CrossRef]
- Perdomo-Morales, R.; Montero-Alejo, V.; Perera, E.; Pardo-Ruiz, Z.; Alonso-Jiménez, E. Phenoloxidase activity in the hemolymph of the spiny lobster Panulirus argus. Fish Shellfish Immunol. 2007, 23, 1187–1195. [Google Scholar] [CrossRef]
- Herrera-Salvatierra, N.; Pascual-Jiménez, C.; Huchin-Mian, J.P.; Lozano-Álvarez, E.; Montero-Muñoz, J.; Briones-Fourzán, P.; Rodríguez-Canul, R. Nutritional and immunological evaluation of juvenile spiny lobsters Panulirus argus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) naturally infected with the PaV1 virus. J. Crustac. Biol. 2019, 39, 162–171. [Google Scholar] [CrossRef]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef]
- Adachi, K.; Hirata, T.; Nishioka, T.; Sakaguchi, M. Hemocyte components in crustaceans convert hemocyanin into a phenoloxidase-like enzyme. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 134, 135–141. [Google Scholar] [CrossRef]
- Galko, M.J.; Krasnow, M.A. Cellular and genetic analysis of wound healing in drosophila larvae. PLoS Biol. 2004, 2, e239. [Google Scholar] [CrossRef] [Green Version]
- Hauton, C. The scope of the crustacean immune system for disease control. J. Invertebr. Pathol. 2012, 110, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jiravanichpaisal, P.; Söderhäll, I.; Cerenius, L.; Söderhäll, K. Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the Crayfish Pacifastacus leniusculus. J. Virol. 2006, 80, 10365–10371. [Google Scholar] [CrossRef] [Green Version]
- Alpuche, J.; Pereyra, A.; Agundis, C.; Rosas, C.; Pascual, C.; Slomianny, M.-C.; Vázquez, L.; Zenteno, E. Purification and characterization of a lectin from the white shrimp Litopenaeus setiferus (Crustacea decapoda) hemolymph. Biochim. Biophys. Acta-Gen. Subj. 2005, 1724, 86–93. [Google Scholar] [CrossRef]
- Luo, T.; Yang, H.; Li, F.; Zhang, X.; Xu, X. Purification, characterization and cDNA cloning of a novel lipopolysaccharide-binding lectin from the shrimp Penaeus monodon. Dev. Comp. Immunol. 2006, 30, 607–617. [Google Scholar] [CrossRef]
- Ma, T.H.T.; Tiu, S.H.K.; He, J.-G.; Chan, S.-M. Molecular cloning of a C-type lectin (LvLT) from the shrimp Litopenaeus vannamei: Early gene down-regulation after WSSV infection. Fish Shellfish Immunol. 2007, 23, 430–437. [Google Scholar] [CrossRef]
- Otálora-Ardila, A.; Herrera, L.G.; Flores-Martínez, J.J.; Welch, K.C. Metabolic cost of the activation of immune response in the fish-eating Myotis (Myotis vivesi): The effects of inflammation and the acute phase response. PLoS ONE 2016, 11, e0164938. [Google Scholar] [CrossRef]
- Hornef, M.W.; Wick, M.J.; Rhen, M.; Normark, S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002, 3, 1033–1040. [Google Scholar] [CrossRef]
- Candia-Zulbarán, R.; Briones-Fourzán, P.; Negrete-Soto, F.; Barradas-Ortiz, C.; Lozano-Álvarez, E. Variability in clinical prevalence of PaV1 in Caribbean spiny lobsters occupying commercial casitas over a large bay in Mexico. Dis. Aquat. Organ. 2012, 100, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Hempel, P. Immune defense, parasite evasion strategies and their relevance for “macroscopic phenomena” such as virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. BioEssays. 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Hernández-Velázquez, I.M.; Zamora-Briseño, J.A.; Pereira-Santana, A.; Huchin-Mian, J.P.; González-Penagos, C.E.; Pérez-Vega, J.A.; Lozano-Álvarez, E.; Briones-Fourzán, P.; Rodríguez-Canul, R. A first glimpse into the transcriptomic changes induced by the PaV1 infection in the gut of Caribbean spiny lobsters, Panulirus argus (Latreille, 1804) (Decapoda: Achelata: Palinuridae). Virus Res. 2022, 311, 198713. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Butler, M.J.; Behringer, D.; Shields, J. Genetic diversity of the Caribbean spiny lobster virus, Panulirus argus virus 1 (PaV1), and the discovery of PaV1 in lobster postlarvae. Aquat. Biol. 2012, 14, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Mydlarz, D.; Jones, E.; Harvell, C. Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Sarathi, M.; Nazeer, A.; Basha, M.; Ravi, C.; Venkatesan, B.; Senthil-Kumar, A.S.; Sahul, H. Clearance of white spot syndrome virus (WSSV) and immunological changes in experimentally WSSV-injected Macrobrachium rosenbergii. Fish Shellfish Immunol. 2008, 25, 222–230. [Google Scholar] [CrossRef]


| Infection Severity | PCR Result | Histopathological Observations | Infection Origin | |
|---|---|---|---|---|
| Nat. | Exp. | |||
| Grade 0 Healthy Lobsters without infection Control treatment of experimental infection | Negative | No aberrant cells with hypertrophied nuclei, no peripheral chromatin or eosinophilic inclusions Hepatopancreas and other tissues appear normal | 54 | 57 |
| Grades 1 Lightly infected | Positive | Few or no infected cells present in hepatopancreas or other organs (1–10 per section) | 10 | 26 |
| Grades 2 Moderately infected | Positive | Fixed phagocytes activated or infected Moderate hemocytic infiltrate Moderate obstruction of hemal sinuses Some infected cells present in organs (1–100 per section) | 12 | 10 |
| Grade 3 Heavily infected | Positive | Hepatopancreatic tubules atrophied Presence of nodulations or granulomas Many infected cells present in spongy connective tissue around organs (>100 per section) | 34 | 8 |
| Source | fd | SS | MS | Pseudo-F | P | Unique Permutations |
|---|---|---|---|---|---|---|
| Carapace length | 1 | 4944.9 | 4944.9 | 21.89 | 0.001 | 999 |
| Origin (O) | 1 | 2703.5 | 2703.5 | 11.95 | 0.001 | 999 |
| Severity (S) | 3 | 6067.4 | 2022.5 | 8.93 | 0.001 | 999 |
| O × S | 3 | 673.9 | 224.63 | 0.99 | 0.452 | 999 |
| Residuals | 202 | 45720 | 226.34 | |||
| Total | 210 | 6011 | ||||
| d.p.i. | Hepatopancreas | Gills | Intestine | Heart | Muscle |
|---|---|---|---|---|---|
| 15 | 1 | 1 | 1 | ||
| 36 | 2 | 1 | 1 | 1 | |
| 57 | 2 | 1 | 1 | 2 | |
| 81 | 3 | 2 | 2 | 2 | |
| 105 | 3 | 2 | 2 | 3 | 1 |
| 132 | 3 | 3 | 2 | 3 | 1 |
| 159 | 3 | 3 | 2 | 3 | 1 |
| 187 | 3 | 3 | 2 | 3 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, C.; Rodríguez-Canul, R.; Huchin-Mian, J.P.; Mascaró, M.; Briones-Fourzán, P.; Lozano-Álvarez, E.; Sánchez, A.; Escalante, K. Immune Response to Natural and Experimental Infection of Panulirus argus Virus 1 (PaV1) in Juveniles of Caribbean Spiny Lobster. Animals 2022, 12, 1951. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12151951
Pascual C, Rodríguez-Canul R, Huchin-Mian JP, Mascaró M, Briones-Fourzán P, Lozano-Álvarez E, Sánchez A, Escalante K. Immune Response to Natural and Experimental Infection of Panulirus argus Virus 1 (PaV1) in Juveniles of Caribbean Spiny Lobster. Animals. 2022; 12(15):1951. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12151951
Chicago/Turabian StylePascual, Cristina, Rossanna Rodríguez-Canul, Juan Pablo Huchin-Mian, Maite Mascaró, Patricia Briones-Fourzán, Enrique Lozano-Álvarez, Ariadna Sánchez, and Karla Escalante. 2022. "Immune Response to Natural and Experimental Infection of Panulirus argus Virus 1 (PaV1) in Juveniles of Caribbean Spiny Lobster" Animals 12, no. 15: 1951. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12151951