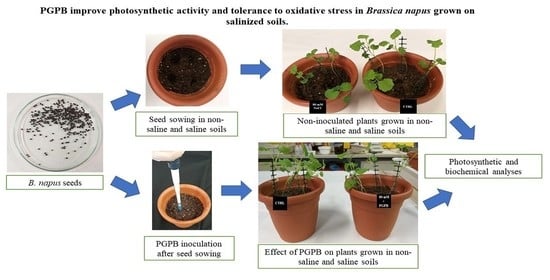
PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Cultures
2.3. Plant Growth Conditions
2.4. Water Content
2.5. Proline Content
2.6. Membrane Injury Index (MII)
2.7. Antioxidant Activity
2.7.1. Phenolic Compounds
2.7.2. Determination of Enzymatic Activities
2.8. Photosynthetic Pigments and Chlorophyll Fluorescence Parameters
2.9. Statistical Analysis
3. Results
3.1. PGPB Halotolerance
3.2. Plant Growth in Saline Conditions
3.3. Effects of PGPB on Canola Exposed to Saline Conditions
3.3.1. Water Content and Osmolyte Synthesis
3.3.2. Membrane Injury Index (MII)
3.3.3. Antioxidant Activity
3.3.4. Photosynthetic Pigments and Chlorophyll Fluorescence Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Khan, K.; Agarwal, P.; Shanware, A.; Sane, V.A. Heterologous expression of two Jatropha aquaporins imparts drought and salt tolerance and improves seed viability in transgenic Arabidopsis thaliana. PLoS ONE 2015, 10, e0128866. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hnilickova, H.; Hnilicka, F.; Martinkovà, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses. J. Plant Growth Reg. 2019, 39, 509–531. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopinski, M.; Małkowski, E. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Pietrini, F.; Zacchini, M.; Iori, V.; Pietrosanti, L.; Ferretti, M.; Massacci, A. Spatial distribution of cadmium in leaves and its impact on photosynthesis: Examples of different strategies in willow and poplar clones. Plant Biol. 2010, 12, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Di Baccio, D.; Iori, V.; Veliksar, S.; Lemanova, N.; Juškaitė, L.; Maruška, A.; Zacchini, M. Investigation on metal tolerance and phytoremoval activity in the poplar hybrid clone “Monviso” under Cu-spiked water: Potential use for wastewater treatment. Sci. Total Environ. 2017, 592, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual exposure to salinity improves tolerance to salt stress in rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef] [Green Version]
- Di Baccio, D.; Pietrini, F.; Bertolotto, P.; Pérez, S.; Barceló, D.; Zacchini, M.; Donati, E. Response of Lemna gibba L. to high and environmentally relevant concentrations of ibuprofen: Removal, metabolism and morpho-physiological traits for biomonitoring of emerging contaminants. Sci. Total Environ. 2017, 584, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Passatore, L.; Fischetti, E.; Carloni, S.; Ferrario, C.; Polesello, S.; Zacchini, M. Evaluation of morpho-physiological traits and contaminant accumulation ability in Lemna minor L. treated with increasing perfluorooctanoic acid (PFOA) concentrations under laboratory conditions. Sci. Total Environ. 2019, 695, 133828. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B. Enzymatic production of biodiesel from canola oil using immobilized lipase. Biomass Bioenergy 2008, 32, 1274–1278. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus tolerance to high saline conditions by seed priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanism of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant. Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.C.; Duan, J.; Di Bernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef] [Green Version]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Yim, W.J.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing ACC deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst. 2021, 1–14. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, A.K.; Berger, B.; Mewis, I.; Ruppel, S. Impact of the PGPB Enterobacter radicincitans DSM 16656 on growth, glucosinolate profile, and immune responses of Arabidopsis thaliana. Microb. Ecol. 2012, 65, 661–670. [Google Scholar] [CrossRef]
- Hussein, A.H.; Joo, J.H. Plant growth-promoting rhizobacteria improved salinity tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 2018, 28, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Dodd, I.C.; Belimov, A.A.; Jiang, F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent development in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Khanghahi, M.; Strafella, S.; Crecchio, C. Changes in photo-protective energy dissipation of photosystem II in response to beneficial bacteria consortium in durum wheat under drought and salinity stresses. Appl. Sci. 2020, 10, 5031. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Leoni, B.; Crecchio, C. Photosynthetic responses of durum wheat to chemical/microbiological fertilization management under salt and drought stresses. Acta. Physiol. Plant 2021, 43, 123. [Google Scholar] [CrossRef]
- Balsanelli, E.; De Baura, V.A.; Pedrosa, F.D.O.; Souza, M.d.S.; Monteiro, R.A. Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae. PLoS ONE 2014, 9, e110392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Gallo, M.; Haegi, A. Characterization and quantification of exocellular polysaccharides in Azospirillum brasilense and Azospirillum lipoferum. Symbiosis 1990, 9, 155–161. [Google Scholar]
- Forni, C.; Haegi, A.; Del Gallo, M.; Grilli Caiola, M. Production of polysaccharides by Arthrobacter globiformis associated with Anabaena azollae in Azolla leaf cavity. FEMS Microb. Lett. 1992, 93, 269–274. [Google Scholar] [CrossRef]
- Forni, C.; Riov, J.; Grilli Caiola, M.; Tel-Or, E. Indole-3-acetic acid (IAA) production by Arthrobacter species isolated from Azolla. J. Gen. Microbiol. 1992, 138, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Pagnani, G.; Rossi, M.; D’Egidio, S.; Del Gallo, M.; Forni, C. Daucus carota L. seed inoculation with a consortium of bacteria improves plant growth, soil fertility status and microbial community. Appl. Sci. 2021, 11, 3274. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J. Appl. Microbiol. 2009, 108, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2012, 58, 427–433. [Google Scholar] [CrossRef]
- Onofre-Lemus, J.; Hernandez-Lucas, I.; Girard, L.; Caballero-Mellado, J. ACC (1-aminocyclopropane-1-carboxylate) deaminase activity, a widespread trait in Burkholderia species, and its growth promoting effect on tomato plants. Appl. Environ. Microbiol. 2009, 75, 6581–6590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New insights into ACC deaminase phylogeny, evolution and evolutionary significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef] [Green Version]
- Nordstedt, N.P.; Jones, M.L. Isolation of rhizosphere bacteria that improve quality and water stress tolerance in greenhouse ornamentals. Front. Plant Sci. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, A.; Chauhan, P.S.; Anandham, R.; Han, G.H.; Sa, T. Isolation, Characterization, and Use for Plant Growth Promotion under Salt Stress, of ACC Deaminase-Producing Halotolerant Bacteria Derived from Coastal Soil. J. Microbiol. Biotechnol. 2010, 27, 1724. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, W.; Cheng, Z.; Heikkila, J.J.; Glick, B.R. The complete genome sequence of the plant growth-promoting bacterium Pseudomonas putida UW4. PLoS ONE 2013, 8, e58640. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stat. Circ. 1950, 347, 1–32. [Google Scholar]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: Separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 1993, 212, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigment of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Bianco, C.; Imperlini, E.; Calogero, R.; Senatore, B.; Amoresano, A.; Carpentieri, A.; Pucci, P.; Defez, R. Indole-3-acetic acid improves Escherichia coli’s defences to stress. Arch. Microbiol. 2006, 185, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djebaili, R.; Pellegrini, M.; Rossi, M.; Forni, C.; Smati, M.; Del Gallo, M.; Kitouni, M. Characterization of plant growth-promoting traits and inoculation effects on Triticum durum of actinomycetes isolates under salt stress conditions. Soil Syst. 2021, 5, 26. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant-plant growth-promoting bacteria: Prospects for mitigating salinity stress in plants. Environ. Exptl. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of salt tolerance and interactive impact of Azotobacter chroococcum and/or Alcaligenes faecalis inoculation on canola (Brassica napus L.) plants grown in saline soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, Y.; Kiyosue, T.; Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997, 38, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, G. Functional consequences of oxidative membrane damage. J. Membr. Biol. 2015, 205, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinty effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Can Turgay, O.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef]
- Pinnola, A.; Staleva-Musto, H.; Capaldi, S.; Ballottari, M.; Bassi, R. Electron transfer between carotenoid and chlorophyll contributes to quenching in the LHCSR1 protein from Physcomitrella patens. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1870–1878. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Bustillos, J.J.; Leyva, L.A.; Hernandez, J.P.; Bacilio, M. Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol. Fertil. Soils 2006, 42, 279–285. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etesami, H. Enhanced phosphorus fertilizer use efficiency with microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Meena, R., Ed.; Springer: Singapore, 2020; pp. 215–245. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.H.; Cruz, C. Current advances in plant growth promoting bacteria alleviating salt stress for sustainable agriculture. Appl. Sci. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Malinská, H.; Pidlisnyuk, V.; Nebeská, D.; Erol, A.; Medžová, A.; Trögl, J. Physiological response of Miscanthus x giganteus to plant growth regulators in nutritionally poor soil. Plants 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Díaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sánchez-Blanco, M.J.; Hernández, J.A. NaCl-induced physiological and biochemical adaptative mechanism in the ornamental Myrtus cummunis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikbal, F.; Hernández, J.A.; Barba-Espín, G.; Koussa, T.; Aziz, A.; Faize, M.; Diaz-Vivancos, P. Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J. Plant Physiol. 2014, 171, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochem. Biophys. Acta 2012, 1817, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzabi, G.; Pinnola, A.; Betterle, N.; Bassi, R.; Alboresi, A. Enhancement of non-photochemical quencing in the Bryophyte Physcomitrella patens during acclimation to salt and osmotic stress. Plant Cell Physiol. 2012, 53, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, H.; Chmeliov, J. Instantaneous switching between different modes of non-photochemical quenching in plants. Consequences for increasing biomass production. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148119. [Google Scholar] [CrossRef] [PubMed]






| Shoots (cm) | Roots (cm) | Fresh Weight (g) | |
|---|---|---|---|
| N.I.C. | 12.68 ± 0.34 a | 8.48 ± 0.37 a | 1.52 ± 0.12 a |
| N.I.S. | 10.71 ± 0.21 b | 8.17 ± 0.57 a | 0.95 ± 0.07 b |
| A.C. | 15.62 ± 0.37 a *** | 9.34 ± 0.49 a | 1.74 ± 0.12 a |
| A.S. | 12.87 ± 0.17 b * | 6.88 ± 0.62 b | 0.9 ± 0.07 b |
| B.C. | 19.73 ± 0.17 a *** | 16.92 ± 0.57 a *** | 3.78 ± 0.09 a *** |
| B.S. | 14.58 ± 0.48 b *** | 8.28 ± 0.31 b | 1.77 ± 0.07 b *** |
| G.C. | 19.24 ± 0.49 a *** | 18.98 ± 0.51 a *** | 4.15 ± 0.17 a *** |
| G.S. | 13.56 ± 0.31 b *** | 8.77 ± 0.29 b | 1.91 ± 0.07 b *** |
| H.C. | 18.66 ± 0.55 a *** | 10.35 ± 0.41 a | 2.65 ± 0.08 a *** |
| H.S. | 13.91 ± 0.62 b *** | 7.64 ± 0.28 b | 0.85 ± 0.08 b |
| U.C. | 15.63 ± 0.21 a *** | 8.6 ± 0.39 a | 1.4 ± 0.09 a |
| U.S. | 13.47 ± 0.24 b *** | 7.41 ± 0.45 a | 1.1 ± 0.08 a |
| Proline (nmoles/mg f.w.) | Phenolic Compounds (µg Chlorogenic Acid eq./g f.w.) | |
|---|---|---|
| N.I.C. | 2.17 ± 0.21 a | 8.57 ± 0.58 a |
| N.I.S. | 6.07 ± 0.28 b | 10.46 ± 0.06 a |
| A.C. | 1.57 ± 0.12 a | 10.57 ± 0.13 a |
| A.S. | 2.95 ± 0.17 a * | 16.67 ± 0.13 b *** |
| B.C. | 0.98 ± 0.09 a * | 8.84 ± 0.18 a |
| B.S. | 0.88 ± 0.18 a *** | 9.67 ± 0.06 a |
| G.C. | 14.62 ± 0.87 a *** | 18.72 ± 0.26 a *** |
| G.S. | 39.68 ± 2.09 b *** | 20.23 ± 1.22 b *** |
| H.C. | 0.69 ± 0.31 a * | 8.50 ± 0.31 a |
| H.S. | 0.75 ± 0.17 a *** | 9.25 ± 0.07 a |
| U. C | 1.54 ± 0.23 a | 8.37 ± 0.17 a |
| U. S | 5.85 ± 0.22 b | 12.77 ± 0.31 b * |
| Total Chlorophylls (µg/g f.w.) | Total Carotenoids (µg/g f.w.) | |
|---|---|---|
| N.I.C. | 265.73 ± 5.02 a | 32.13 ± 0.77 a |
| N.I.S. | 301.54 ± 6.49 b | 37.81 ± 0.79 b |
| A.C. | 315.13 ± 17.98 a * | 34.72 ± 2.1 a |
| A.S. | 414.42 ± 22.07 b ** | 53.45 ± 2.31 b ** |
| B.C. | 282.33 ± 8.19 a | 35.59 ± 1.5 a |
| B.S. | 221.82 ± 15.70 b * | 42.25 ± 1.31 b * |
| G.C. | 349.58 ± 18.88 a * | 43.55 ± 3.5 a * |
| G.S. | 322.53 ± 15.01 a | 41.15 ± 2.06 a |
| H.C. | 227.82 ± 9.27 a * | 30.97 ± 0.89 a |
| H.S. | 278.47 ± 19.36 b | 44.69 ± 2.96 b * |
| U. C | 379.51 ± 49.4 a * | 51.51 ± 5.7 a *** |
| U. S | 415.59 ± 19.11 a ** | 51.43 ± 2.47 a ** |
| Fv/Fm (r.u.) | ΦPSII (r.u.) | NPQ (r.u.) | ETR (μmol electrons m−2 s−1) | |
|---|---|---|---|---|
| N.I.C. | 0.8086 ± 0.0019 a | 0.488 ± 0.004 a | 0.276 ± 0.015 a | 11.29 ± 0.02 a |
| N.I.S. | 0.8049 ± 0.0069 a | 0.440 ± 0.004 b | 0.306 ± 0.009 a | 10.45 ± 0.11 b |
| A.C. | 0.8150 ± 0.0015 a | 0.499 ± 0.004 a | 0.188 ± 0.009 a * | 11.62 ± 0.15 a |
| A.S. | 0.8198 ± 0.0001 b * | 0.488 ± 0.004 a * | 0.272 ± 0.012 b | 11.30 ± 0.17 a * |
| B.C. | 0.8195 ± 0.0010 a | 0.482 ± 0.005 a | 0.172 ± 0.006 a * | 11.17 ± 0.08 a |
| B.S. | 0.8241 ± 0.0009 b * | 0.503 ± 0.008 a * | 0.228 ± 0.020 b * | 11.60 ± 0.21 a * |
| G.C. | 0.8098 ± 0.0029 a | 0.481 ± 0.016 a | 0.248 ± 0.016 a | 10.92 ± 0.60 a |
| G.S. | 0.8182 ± 0.0017 a * | 0.488 ± 0.003 a * | 0.252 ± 0.016 a | 11.35 ± 0.29 a * |
| H.C. | 0.7979 ± 0.0080 a | 0.467 ± 0.003 a | 0.208 ± 0.010 a * | 10.87 ± 0.07 a |
| H.S. | 0.8186 ± 0.0014 b * | 0.469 ± 0.004 a * | 0.345 ± 0.028 b | 10.87 ± 0.06 a * |
| U. C | 0.8160 ± 0.0023 a | 0.511 ± 0.001 a | 0.164 ± 0.010 a * | 11.41 ± 0.08 a |
| U. S | 0.8200 ± 0.0016 a * | 0.454 ± 0.009 b | 0.239 ± 0.031 a * | 10.85 ± 0.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311442
Rossi M, Borromeo I, Capo C, Glick BR, Del Gallo M, Pietrini F, Forni C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Applied Sciences. 2021; 11(23):11442. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311442
Chicago/Turabian StyleRossi, Massimiliano, Ilaria Borromeo, Concetta Capo, Bernard R. Glick, Maddalena Del Gallo, Fabrizio Pietrini, and Cinzia Forni. 2021. "PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils" Applied Sciences 11, no. 23: 11442. https://0-doi-org.brum.beds.ac.uk/10.3390/app112311442