Rapid-Response Magnetic Enrichment Strategy for Significantly Improving Sensitivity of Multiplex PCR Analysis of Pathogenic Listeria Species
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bacterial Strains
2.3. Preparation and Characterization of PEI-MNPs
2.4. Procedure of Bacteria Separation
2.5. Effects of Bacterial Capture
2.6. DNA Extraction
2.7. mPCR Analysis
2.8. Method Performance in Artificially Contaminated Lettuce
2.9. Statistical Analysis
3. Results and Discussion
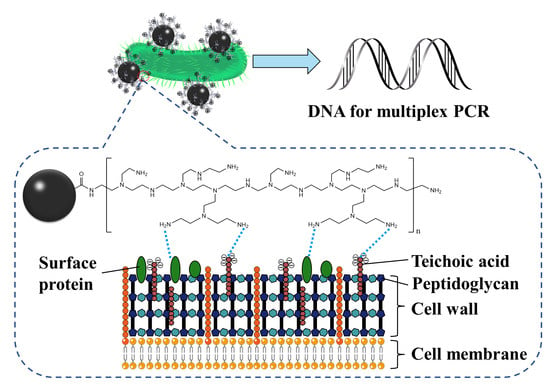
3.1. Principles of the Proposed Strategy
3.2. Characterization of PEI-MNPs
3.3. Bacteria Captured by PEI-MNPs
3.4. Factors Affecting the Bacterial Capture
3.5. Optimization of Enrichment Parameters
3.6. Evaluation of Method Performance in Artificially Contaminated Lettuce
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, D.; Zhuang, Z.; Wang, Z.; Li, S.; Zhong, H.; Liu, Z.; Guo, Z.; Zhang, W. Black phosphorus-Au filter paper-based three-dimensional SERS substrate for rapid detection of foodborne bacteria. Appl. Surf. Sci. 2019, 497, 143825. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Architecture and Viability of the Biofilms Formed by Nine Listeria Strains on Various Hydrophobic and Hydrophilic Materials. Appl. Sci. 2019, 9, 5256. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.E.; Kang, T.; Jenkins, D.; Li, Y.; Wall, M.M.; Jun, S. A single-walled carbon nanotubes-based electrochemical impedance immunosensor for on-site detection of Listeria monocytogenes. J. Food Sci. 2022, 87, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, T.; Wang, X.; Liu, Q.; Dong, Q. Probabilistic model for estimating Listeria monocytogenes concentration in cooked meat products from presence/absence data. Food Res. Int. 2020, 131, 109040. [Google Scholar] [CrossRef]
- Kljujev, I.; Raicevic, V.; Jovicic-Petrovic, J.; Vujovic, B.; Mirkovic, M.; Rothballer, M. Listeria monocytogenes—Danger for health safety vegetable production. Microb. Pathog. 2018, 120, 23–31. [Google Scholar] [CrossRef]
- Olea-Rodríguez, M.d.l.Á.; Chombo-Morales, P.; Nuño, K.; Vázquez-Paulino, O.; Villagrán-de la Mora, Z.; Garay-Martínez, L.E.; Castro-Rosas, J.; Villarruel-López, A.; Torres-Vitela, M.R. Microbiological Characteristics and Behavior of Staphylococcus aureus, Salmonella spp., Listeria monocytogenes and Staphylococcal Toxin during Making and Maturing Cotija Cheese. Appl. Sci. 2021, 11, 8154. [Google Scholar] [CrossRef]
- Jamali, H.; Paydar, M.; Ismail, S.; Looi, C.Y.; Wong, W.F.; Radmehr, B.; Abedini, A. Prevalence, antimicrobial susceptibility and virulotyping of Listeria species and Listeria monocytogenes isolated from open-air fish markets. BMC Microbiol. 2015, 15, 144. [Google Scholar] [CrossRef] [Green Version]
- de Niederhäusern, S.; Bondi, M.; Camellini, S.; Sabia, C.; Messi, P.; Iseppi, R. Plant Extracts for the Control of Listeria monocytogenes in Meat Products. Appl. Sci. 2021, 11, 10820. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union one health 2020 zoonoses report. EFSA J. 2021, 19, e06971. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- Morganti, M.; Scaltriti, E.; Cozzolino, P.; Bolzoni, L.; Casadei, G.; Pierantoni, M.; Foni, E.; Pongolini, S. Processing-Dependent and Clonal Contamination Patterns of Listeria monocytogenes in the Cured Ham Food Chain Revealed by Genetic Analysis. Appl. Environ. Microbiol. 2016, 82, 822–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Techathuvanan, C.; D’Souza, D.H. Propidium monoazide for viable Salmonella enterica detection by PCR and LAMP assays in comparison to RNA-based RT-PCR, RT-LAMP, and culture-based assays. J. Food Sci. 2020, 85, 3509–3516. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Xing, D. Simultaneous detection of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes using oscillatory-flow multiplex PCR. Microchim. Acta 2011, 173, 503–512. [Google Scholar] [CrossRef]
- Germini, A.; Masola, A.; Carnevali, P.; Marchelli, R. Simultaneous detection of Escherichia coli O175:H7, Salmonella spp., and Listeria monocytogenes by multiplex PCR. Food Control 2009, 20, 733–738. [Google Scholar] [CrossRef]
- Xie, G.; Yu, S.; Li, W.; Mu, D.; Aguilar, Z.P.; Xu, H. Simultaneous detection of Salmonella spp., Pseudomonas aeruginosa, Bacillus cereus, and Escherichia coli O157:H7 in environmental water using PMA combined with mPCR. J. Microbiol. 2020, 58, 668–674. [Google Scholar] [CrossRef]
- Kotsiri, Z.; Vantarakis, A.; Rizzotto, F.; Kavanaugh, D.; Ramarao, N.; Vidic, J. Sensitive Detection of E. coli in Artificial Seawater by Aptamer-Coated Magnetic Beads and Direct PCR. Appl. Sci. 2019, 9, 5392. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Lin, J.; Gan, C.; Wang, Y.; Wang, D.; Xiong, Y.; Lai, W.; Li, Y.; Wang, M. A sensitive impedance biosensor based on immunomagnetic separation and urease catalysis for rapid detection of Listeria monocytogenes using an immobilization-free interdigitated array microelectrode. Biosens. Bioelectron. 2015, 74, 504–511. [Google Scholar] [CrossRef]
- Meng, X.; Yang, G.; Li, F.; Liang, T.; Lai, W.; Xu, H. Sensitive Detection of Staphylococcus aureus with Vancomycin-Conjugated Magnetic Beads as Enrichment Carriers Combined with Flow Cytometry. ACS Appl. Mater. Interfaces 2017, 9, 21464–21472. [Google Scholar] [CrossRef]
- Yang, G.; Huang, M.; Wang, Y.; Chen, G.; Zhao, Y.; Xu, H. Streptavidin-exposed magnetic nanoparticles for lectin magnetic separation (LMS) of Staphylococcus aureus prior to three quantification strategies. Microchim. Acta 2019, 186, 813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, R.; Liu, W.; Liu, H.; Zhou, X.; Xing, D. Rapid and visual detection of Listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosens. Bioelectron. 2016, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Wang, Y.; Chen, G.; Liu, J.; Wang, Z.; Xu, H. Poly-l-lysine-functionalized magnetic beads combined with polymerase chain reaction for the detection of Staphylococcus aureus and Escherichia coli O157:H7 in milk. J. Dairy Sci. 2021, 104, 12342–12352. [Google Scholar] [CrossRef]
- Cremin, K.; Jones, B.A.; Teahan, J.; Meloni, G.N.; Perry, D.; Zerfass, C.; Asally, M.; Soyer, O.S.; Unwin, P.R. Scanning Ion Conductance Microscopy Reveals Differences in the Ionic Environments of Gram-Positive and Negative Bacteria. Anal. Chem. 2020, 92, 16024–16032. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, M.; Xu, X.; Zhang, B.; Duan, Y.; Wei, Y.; Wang, J.; Mingyang, L. Rapid photocatalytic inactivation of E. coli by polyethyleneimine grafted O-doped g-C3N4: Synergetic effects of the boosted reactive oxygen species production and adhesion performance. Appl. Surf. Sci. 2022, 573, 151496. [Google Scholar] [CrossRef]
- Tao, T.; Chen, Q.; Bie, X.; Lu, F.; Lu, Z. Mining of novel species-specific primers for PCR detection of Listeria monocytogenes based on genomic approach. World J. Microbiol. Biotechnol. 2015, 31, 1955–1966. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, X.; Xiong, S.; Xu, H.; Aguilar, Z.P.; Xiong, Y. Large-volume immunomagnetic separation combined with multiplex PCR assay for simultaneous detection of Listeria monocytogenes and Listeria ivanovii in lettuce. Food Control 2016, 59, 601–608. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Yang, C.Y.; Li, C.; Ho, Y.C.; Lin, C.K.; Tsen, H.Y. Identification of Bacillus spp., Escherichia coli, Salmonella spp., Staphylococcus spp. and Vibrio spp. with 16S ribosomal DNA-based oligonucleotide array hybridization. Int. J. Food Microbiol. 2006, 107, 131–137. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, J.; Feng, X.; Xie, G.; Zhang, W.; Xu, H. Rapid enrichment and detection of Staphylococcus aureus in milk using polyethyleneimine functionalized magnetic nanoparticles. Microchem. J. 2022, 178, 107388. [Google Scholar] [CrossRef]
- Bierne, H.; Cossart, P. Listeria monocytogenes Surface Proteins: From Genome Predictions to Function. Microbiol. Mol. Biol. Rev. 2007, 71, 377–397. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ma, J.; Ruan, J.; Zhuang, X. Using Positively Charged Magnetic Nanoparticles to Capture Bacteria at Ultralow Concentration. Nanoscale Res. Lett. 2019, 14, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Liu, F.; Shan, C.; Tong, M.; Hou, Y. Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res. 2014, 50, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.C.; Zhao, G.; Saha, K.; Phillips, R.L.; Li, X.; Miranda, O.R.; Rotello, V.M.; El-Sayed, M.A.; Schmidt-Krey, I.; Bunz, U.H. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc. 2012, 134, 6920–6923. [Google Scholar] [CrossRef] [PubMed]
- Thiramanas, R.; Laocharoensuk, R. Competitive binding of polyethyleneimine-coated gold nanoparticles to enzymes and bacteria: A key mechanism for low-level colorimetric detection of gram-positive and gram-negative bacteria. Microchim. Acta 2015, 183, 389–396. [Google Scholar] [CrossRef]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in Fresh Produce: Outbreaks, Prevalence and Contamination Levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]





| Target Bacteria | Target Gene | Primer | Sequences (5′-3′) | Length (bp) | Reference |
|---|---|---|---|---|---|
| L. monocytogenes | LMOf2365_2721 | lm13-F | GTTCGTCGGTCCGTGGTA | 583 | [26] |
| lm13-R | TTGGCAAGCAAGCAGTTCA | ||||
| L. ivanovii | iacta | iacta-F | TAGAGAATGGCGAGGAGGAGTA | 311 | [27] |
| iacta-R | TCCGGGACATTTGCACT | ||||
| Bacteria DNA | 16s rRNA | 16s rRNA-F | CCTACGGGAGGCAGCAGT | 475 | [28] |
| 16s rRNA-R | CGTTTACGGCGTGGACTAC |
| Bacterial Strains | Source | Strain ID | Results of mPCR | ||
|---|---|---|---|---|---|
| lm13 | iacta | 16s rRNA | |||
| Listeria monocytogenes | ATCC 1 | 19115 | + | − | + |
| CMCC 2 | 54001 | + | − | + | |
| Listeria ivanovii | ATCC | 19119 | − | + | + |
| Listeria innocua | ATCC | 11288 | − | − | + |
| Listeria seeligeri | ATCC | 35967 | − | − | + |
| Listeria grayi | ATCC | 25401 | − | − | + |
| Listeria welshimeri | ATCC | 35897 | − | − | + |
| Staphyloccocus aureus | CMCC | 26001 | − | − | + |
| Cronobacter sakazakii | ATCC | 29544 | − | − | + |
| Escherichia coli | ATCC | 25922 | − | − | + |
| Escherichia coli O157:H7 | ATCC | 43888 | − | − | + |
| Bacillus cereus | JX-CDC 3 | JDZ102Y | − | − | + |
| Salmonella Enteritidis | ATCC | 13076 | − | − | + |
| Salmonella Typhimurium | ATCC | 13311 | − | − | + |
| Bacillus subtilis | CMCC | 63501 | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Bai, X.; Wang, K.; Sun, Y.; Xu, H. Rapid-Response Magnetic Enrichment Strategy for Significantly Improving Sensitivity of Multiplex PCR Analysis of Pathogenic Listeria Species. Appl. Sci. 2022, 12, 6415. https://0-doi-org.brum.beds.ac.uk/10.3390/app12136415
Xiao F, Bai X, Wang K, Sun Y, Xu H. Rapid-Response Magnetic Enrichment Strategy for Significantly Improving Sensitivity of Multiplex PCR Analysis of Pathogenic Listeria Species. Applied Sciences. 2022; 12(13):6415. https://0-doi-org.brum.beds.ac.uk/10.3390/app12136415
Chicago/Turabian StyleXiao, Fangbin, Xuekun Bai, Keyu Wang, Yifan Sun, and Hengyi Xu. 2022. "Rapid-Response Magnetic Enrichment Strategy for Significantly Improving Sensitivity of Multiplex PCR Analysis of Pathogenic Listeria Species" Applied Sciences 12, no. 13: 6415. https://0-doi-org.brum.beds.ac.uk/10.3390/app12136415