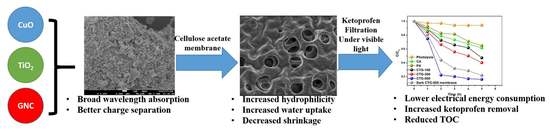
Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of TiO2, CuO/TiO2 Nanoparticles and Graphitic Carbon Nitride (GCN)
2.3. Preparation of 1% CuO/TiO2@GCN Photocatalysts
2.4. Preparation of CA Membranes
2.5. Preparation of CuO/TiO2@GCN-CA Photocatalytic Membranes
2.6. Materials Characterization
2.7. Flux and Antifouling Properties
2.8. Evaluation of the Efficiency of the Photocatalytic Membrane
3. Results and Discussion
3.1. Material Characterizations
3.2. PXRD Analysis
3.3. XPS Analysis
3.4. FTIR Analysis
3.5. Membrane Characterizations
SEM Analysis of the Membranes
3.6. Surface Hydrophilicity and Water Uptake Evaluation
Surface Hydrophilicity
3.7. Water Uptake
3.8. Permeability Evaluation
Porosity and Shrinkage of the Membrane
3.9. Pure Water Permeation and Membrane Recyclability Evaluation
3.10. The Photocatalytic Performance of Modified CA Membranes
3.10.1. Photodegradation of KP in Deionized Water
3.10.2. Photodegradation of KP in Drinking and Groundwater Using CTG–500 and Costs Associated with the Process
3.10.3. Photocatalytic Degradation of KP Drinking and Groundwater: Antifouling and Regeneration Studies in Drinking and Groundwater
3.11. Photocatalytic Mechanism
The Effect Scavengers
3.12. Proposed Mechanism
3.13. Proposed Degradation Pathway of KP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuvarega, A.T.; Khumalo, N.P.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Chi, L.; Qian, Y.; Guo, J.; Wang, X.; Arandiyan, H.; Jiang, Z. Novel g-C3N4/TiO2/PAA/PTFE ultrafiltration mem-brane enabling enhanced antifouling and exceptional visible-light photocatalytic self-cleaning. Catal. Today 2019, 335, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Nyamutswa, L.; Zhu, B.; Collins, S.F.; Navaratna, D.; Duke, M.C. Light conducting photocatalytic membrane for chemical-free fouling control in water treatment. J. Membr. Sci. 2020, 604, 118018. [Google Scholar] [CrossRef]
- Fane, A.G.; Xi, W.; Rong, W. Interface Science in Drinking Water Treatment-Theory and Application, Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 10, pp. 109–132. [Google Scholar]
- Diallo, M.S. Water Treatment by Dendrimer-Enhanced Filtration: Principles and Applications. In Nanotechnology Applications for Clean Water; William Andrew Publishing: New York, NY, USA, 2014; pp. 143–155. [Google Scholar] [CrossRef]
- Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Zhu, Z.; Feng, Y.; Wu, C. Changing conventional blending photocatalytic membranes (BPMs): Focus on improving photocatalytic performance of Fe3O4/g-C3N4/PVDF membranes through magnetically induced freezing casting method. Chem. Eng. J. 2019, 365, 405–414. [Google Scholar] [CrossRef]
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 Photocatalytic Membranes with a Hierarchical Mesoporous Multilayer Structure: Synthesis, Characterization, and Multifunction. Adv. Funct. Mater. 2006, 16, 1067–1074. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; da Costa, J.C.D. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Hlekelele, L.; Durbach, S.H.; Chauke, V.P.; Dziike, F.; Franklyn, P.J. Resin-gel incorporation of high concentrations of W6+ and Zn2+ into TiO2-anatase crystal to form quaternary mixed-metal oxides: Effect on the a lattice parameter and photodegradation efficiency. RSC Adv. 2019, 9, 36875–36883. [Google Scholar] [CrossRef] [Green Version]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of gra-phene-TiO2 nanotube composites with enhanced photocatalytic activity. Acs Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Alias, S.H.; Mohamed, N.N.; Loon, L.W.; Chandren, S. Synthesis of carbon self-doped titanium dioxide and its activity in the photocatalytic oxidation of styrene under visible light irradiation. Malays. J. Fundam. Appl. Sci. 2019, 15, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Kiyonaga, T.; Naya, S.-I. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium(iv) dioxide. Chem. Soc. Rev. 2009, 38, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Dziike, F.; Franklyn, P.J.; Hlekelele, L.; Durbach, S.H. Synthesis of carbon nanofibers over lanthanum supported on radially aligned nanorutile: A parametric study. Diam. Relat. Mater. 2019, 99, 107519. [Google Scholar] [CrossRef]
- de Brito, J.F.; Tavella, F.; Genovese, C.; Ampelli, C.; Zanoni, M.V.B.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2 nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.-H.; Ma, S.-Q.; Du, X.-D.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr(VI) reduction performance under white light. Chem. Eng. J. 2019, 375, 121944. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Feng, Q.; Chen, X.; Hu, Z. Visible light photocatalytic degradation of MB using UiO-66/g-C3N4 heterojunction nanocatalyst. Chemosphere 2018, 212, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, D.; Ghugal, S.G.; Umare, S.S.; Banavoth, M. Extended π-conjugative n-p type homostructural graphitic carbon nitride for photodegradation and charge-storage applications. Sci. Rep. 2019, 9, 7186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H. Construction of an all-solid-state Z-scheme photocatalyst based on graphite carbon nitride and its enhancement to catalytic activity. Environ. Sci. Nano 2018, 5, 599–615. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, B.; Wang, W.; Jiang, C.; Yu, J. Ag2CrO4/g-C3N4/graphene oxide ternary nanocomposite Z-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2018, 231, 368–380. [Google Scholar] [CrossRef]
- Hlekelele, L.; Nomadolo, N.E.; Setshedi, K.Z.; Mofokeng, L.E.; Chetty, A.; Chauke, V.P. Synthesis and characterization of polyaniline, polypyrrole and zero-valent iron-based materials for the adsorptive and oxidative removal of bisphenol-A from aqueous solution. RSC Adv. 2019, 9, 14531–14543. [Google Scholar] [CrossRef] [Green Version]
- Rajbongshi, B.M.; Samdarshi, S.K.; Boro, B. Multiphasic bi-component TiO2–ZnO nanocomposite: Synthesis, characterization and investigation of photocatalytic activity under different wavelengths of light irradiation. J. Mater. Sci. Mater. Electron. 2015, 26, 377–384. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.-M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B Environ. 1998, 18, 281–291. [Google Scholar] [CrossRef]
- Abdelhaleem, A.; Chu, W. Monuron photodegradation using peroxymonosulfate activated by non-metal-doped TiO2 under visible LED and the modeling via a parallel-serial kinetic approach. Chem. Eng. J. 2018, 338, 411–421. [Google Scholar] [CrossRef]
- Abdelhaleem, A.; Chu, W. Photodegradation of 4-chlorophenoxyacetic acid under visible LED activated N-doped TiO2 and the mechanism of stepwise rate increment of the reused catalyst. J. Hazard. Mater. 2017, 338, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhou, J.; Bao, J.; Feng, Y. Photocatalytic activity of (copper, nitrogen)-codoped titanium dioxide nano-particles. J. Am. Ceram. Soc. 2008, 91, 1369–1371. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Lu, S.; Pan, Y.; Gu, H. Citrate/F− assisted phase control synthesis of TiO2 nanostructures and their photocatalytic properties. RSC Adv. 2015, 5, 74230–74237. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z. Porous g-C3N4 with enhanced adsorption and visible-light photocatalytic performance for removing aqueous dyes and tetracycline hydrochloride. Chin. J. Chem. Eng. 2018, 26, 753–760. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhang, J.; Sun, J. Onion-like carbon modified porous graphitic carbon nitride with excellent photocatalytic activities under visible light. Ceram. Int. 2016, 42, 18116–18123. [Google Scholar] [CrossRef]
- Adekoya, D.O.; Tahir, M.; Amin, N.A.S. g-C3N4/(Cu/TiO2) nanocomposite for enhanced photoreduction of CO2 to CH3OH and HCOOH under UV/visible light. J. CO2 Util. 2017, 18, 261–274. [Google Scholar] [CrossRef]
- Nair, A.K.; JagadeeshBabu, P.E. Ag-TiO2 nanosheet embedded photocatalytic membrane for solar water treatment. J. Environ. Chem. Eng. 2017, 5, 4128–4133. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Feng, Y. Bimetallic Au/Ag decorated TiO2 nanocom-posite membrane for enhanced photocatalytic degradation of tetracycline and bactericidal efficiency. Appl. Surf. Sci. 2019, 487, 1008–1017. [Google Scholar] [CrossRef]
- Desa, A.L.; Hairom, N.H.H.; Ng, L.Y.; Ng, C.Y.; Ahmad, M.K.; Mohammad, A.W. Industrial textile wastewater treatment via membrane photocatalytic reactor (MPR) in the presence of ZnO-PEG nanoparticles and tight ultrafiltration. J. Water Process Eng. 2019, 31, 100872. [Google Scholar] [CrossRef]
- Moustakas, N.; Katsaros, F.; Kontos, A.; Romanos, G.; Dionysiou, D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Hong, C.-H.; Ki, S.-J.; Jeon, J.-H.; Che, H.-L.; Park, I.-K.; Kee, C.-D.; Oh, I. Electroactive bio-composite actuators based on cellulose acetate nanofibers with specially chopped polyaniline nanoparticles through electrospinning. Compos. Sci. Technol. 2013, 87, 135–141. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Hlekelele, L.; Franklyn, P.J.; Dziike, F.; Durbach, S.H. TiO2 composited with carbon nanofibers or nitrogen-doped carbon nanotubes synthesized using coal fly ash as a catalyst: Bisphenol-A photodegradation efficiency evaluation. New J. Chem. 2018, 42, 4531–4542. [Google Scholar] [CrossRef]
- Pareek, S.; Quamara, J.K. Dielectric and optical properties of graphitic carbon nitride–titanium dioxide nanocomposite with enhanced charge seperation. J. Mater. Sci. 2017, 53, 604–612. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Jiang, X.X.; De Hu, X.; Tarek, M.; Saravanan, P.; Alqadhi, R.; Chin, S.Y.; Khan, M.R. Tailoring the properties of g-C3N4 with CuO for enhanced photoelectrocatalytic CO2 reduction to methanol. J. CO2 Util. 2020, 40, 101222. [Google Scholar] [CrossRef]
- Shi, Q.; Ping, G.; Wang, X.; Xu, H.; Li, J.; Cui, J.; Abroshan, H.; Ding, H.; Li, G. CuO/TiO2 heterojunction composites: An efficient photocatalyst for selective oxidation of methanol to methyl formate. J. Mater. Chem. A 2019, 7, 2253–2260. [Google Scholar] [CrossRef]
- Dar, M.; Ahsanulhaq, Q.; Kim, Y.; Sohn, J.; Kim, W.B.; Shin, H. Versatile synthesis of rectangular shaped nanobat-like CuO nanostructures by hydrothermal method; structural properties and growth mechanism. Appl. Surf. Sci. 2009, 255, 6279–6284. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Q.; Huang, L.; Feng, H.; Zhang, C.; Xu, H.; Wang, M. Toward Efficient Preconcentrating Photocatalysis: 3D g-C3N4 Monolith with Isotype Heterojunctions Assembled from Hybrid 1D and 2D Nanoblocks. ACS Appl. Mater. Interfaces 2019, 11, 31934–31942. [Google Scholar] [CrossRef] [PubMed]
- Mathumba, P.; Maziya, K.; Kuvarega, A.T.; Dlamini, L.N.; Malinga, S.P. Photocatalytic degradation of a basic dye in water by nanostructured HPEI/TiO2 containing membranes. Water SA 2020, 46, 500–505. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Li, X.; Dai, H. An antibacterial composite film based on cellulose acetate/TiO2 nanoparticles. New J. Chem. 2020, 44, 20751–20758. [Google Scholar] [CrossRef]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and characterization of TiO2-PVDF/PMMA blend membranes using an alternative non-toxic solvent for UF/MF and photocatalytic application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef] [Green Version]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation ultrafine L-Methionine (C, N, S triple doped)-TiO2-ZnO nanoparticles and their photocatalytic performance for fouling alleviation in PES nanocomposite membrane. Compos. Part B Eng. 2019, 176, 107158. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, H.; Min, X.; Zhu, X. High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment. J. Appl. Polym. Sci. 2020, 137, 48488. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Braeken, L.; Liu, T.-Y.; Luis, P.; Wang, X.-L.; Van Der Bruggen, B. Remarkable Anti-Fouling Performance of TiO2-Modified TFC Membranes with Mussel-Inspired Polydopamine Binding. Appl. Sci. 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Khurram, R.; Javed, A.; Ke, R.; Lena, C.; Wang, Z. Visible Light-Driven GO/TiO2-CA Nano-Photocatalytic Membranes: Assessment of Photocatalytic Response, Antifouling Character and Self-Cleaning Ability. Nanomaterials 2021, 11, 2021. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, B.; Huang, C.-P.; Pan, S.-Y.; Wu, P.; Dang, Z.; Chiang, P.-C. Performance evaluation of integrated adsorption-nanofiltration system for emerging compounds removal: Exemplified by caffeine, diclofenac and octylphenol. J. Environ. Manag. 2019, 231, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Vogel, D.; Khan, S. Characterising humic acid fouling of nanofiltration membranes using bisphenol A as a molecular indicator. Water Res. 2008, 42, 4049–4058. [Google Scholar] [CrossRef]
- Hlekelele, L.; Franklyn, P.J.; Tripathi, P.K.; Durbach, S.H. Morphological and crystallinity differences in nitrogen-doped carbon nanotubes grown by chemical vapour deposition decomposition of melamine over coal fly ash. RSC Adv. 2016, 6, 76773–76779. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wei, J.; Liu, K.; Liu, N.; Zhou, B. Adsorption of Bisphenol a Based on Synergy between Hydrogen Bonding and Hydrophobic Interaction. Langmuir 2014, 30, 13861–13868. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Xi, H.; Li, Q.; Shen, M.; Ying, G.; Zhang, J. Polydopamine functionalized cellulose-MXene composite aerogel with superior adsorption of methylene blue. Cellulose 2021, 28, 4281–4293. [Google Scholar] [CrossRef]












| Membrane ID | CA:DMSO Ratio | PVP/wt% | 1% CuO/TiO2@GCN (9:1) Content/wt% |
|---|---|---|---|
| CA | 15:83 | 2.00 | 0.00 |
| CTG–100 | 15:83 | 2.00 | 0.10 |
| CTG–300 | 15:83 | 2.00 | 0.30 |
| CTG–500 | 15:83 | 2.00 | 0.50 |
| Membrane ID | Water Uptake/% | Porosity/% | Shrinkage/% |
|---|---|---|---|
| CA | 72.00 | 79.42 | 31.85 |
| CTG–100 | 76.93 | 83.37 | 18.65 |
| CTG–300 | 78.06 | 84.25 | 17.68 |
| CTG–500 | 80.53 | 86.14 | 13.83 |
| Membrane ID | Rate Constant/h−1 | Degradation Efficiency/% | EEC Costs/m3 |
|---|---|---|---|
| CTG–100 | 0.141 | 46.1 | 5.92 × 104 |
| CTG–300 | 0.181 | 61.5 | 4.63 × 104 |
| CTG–500 | 0.390 | 83.7 | 2.18 × 104 |
| CTG–500 (in darkness) | 0.315 | 78.1 | 2.69 × 104 |
| CA | 0.095 | 38.3 | 8.75 × 104 |
| PA supporting membrane | 0.085 | 35.3 | 9.78 × 104 |
| Photolysis | 0.012 | 5.6 | 6.88 × 105 |
| Membrane ID | Rate Constant/h−1 | Degradation Efficiency/% | EEC/kWh/m3 |
|---|---|---|---|
| CTG–500 (Groundwater) | 0.508 | 94.8 | 1.69 × 104 |
| CTG–500 (Drinking water) | 0.568 | 94.0 | 1.52 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofokeng, L.E.; Hlekelele, L.; Moma, J.; Tetana, Z.N.; Chauke, V.P. Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater. Appl. Sci. 2022, 12, 1649. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031649
Mofokeng LE, Hlekelele L, Moma J, Tetana ZN, Chauke VP. Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater. Applied Sciences. 2022; 12(3):1649. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031649
Chicago/Turabian StyleMofokeng, Lethula E., Lerato Hlekelele, John Moma, Zikhona N. Tetana, and Vongani P. Chauke. 2022. "Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater" Applied Sciences 12, no. 3: 1649. https://0-doi-org.brum.beds.ac.uk/10.3390/app12031649