Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented S. maxima Fermentation
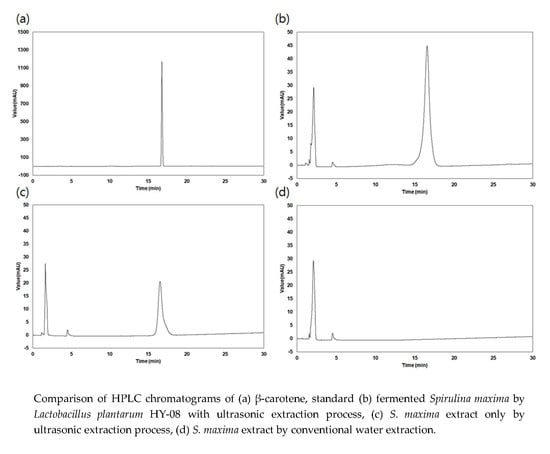
2.2. Determination of β-Carotene Content in the Extracts
2.3. Measurement of the DPPH Free Radical-Scavenging Activity of the Extracts
2.4. Measurement of the Neuroprotective Activity of the Extracts
2.5. Determination of the Inhibition of ROS and Ca2+ Production from HT22 Cells
2.6. Measurement of Glutathione Peroxidase and Glutathione Reductase Activities via Treatment of the Extracts
2.7. Western Blot Analysis of BDNF (Brain-Derived Neurotrophic Factor) and p-CREB (cAMP-Responsive Element-Binding Protein) Gene Expression Induced by the Extract
2.8. Statistical Analysis
3. Results
3.1. Extraction Yield and β-Carotene Content of the Extracts
3.2. Comparison of Antioxidant and Neuroprotective Activities of β-Carotene and the Fermented S. maxima Extract
3.3. Neuroprotective Effects of the Fermented S. maxima Extract against Brain Oxidative Stress
3.4. Enhancement of BDNF/p-CREB Signaling Pathways in Glutamate-Induced, Oxidatively Stressed HT22 Cells with the Fermented S. maxima Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Citron, M. Alzheimer’s disease: Treatments in discovery and development. Nat. Neurosci. 2002, 5, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.R.; Iversen, S.D. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behav. Brain Res. 1993, 57, 143–153. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-amyloid peptide. J. Alzheimers Dis. 2010, 19, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hannula, M.J.; Myöhänen, T.T.; Tenorio-Laranga, J.; Männistö, P.T.; Garcia-Horsman, J.A. Prolyl oligopeptidase colocalizes with α-synuclein, β-amyloid, tau protein and astroglia in the post-mortem brain samples with Parkinson’s and Alzheimer’s diseases. Neuroscience 2013, 242, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Toide, K.; Shinoda, M.; Fujiwara, T.; Iwamoto, Y. Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on spatial memory and central cholinergic neurons in aged rats. Pharmacol. Biochem. Behav. 1997, 56, 427–434. [Google Scholar] [CrossRef]
- Fukunari, A.; Kato, A.; Sakai, Y.; Yoshimoto, T.; Ishiura, S.; Suzuki, K.; Nakajima, T. Colocalization of prolyl endopeptidase and amyloid beta-peptide in brains of senescence-accelerated mouse. Neurosci. Lett. 1994, 176, 201–204. [Google Scholar] [CrossRef]
- Takeda, A.; Loveman, E.; Clegg, A.; Kirby, J.; Picot, J.; Payne, E.; Green, C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Rodda, J.; Morgan, S.; Walker, Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int. Psychogeriatr. 2009, 21, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Kim, D.H.; Moon, Y.S.; Jung, J.S.; Ahn, E.M.; Baek, N.I.; Song, D.K. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef]
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The Efficacy of Ginkgo biloba on cognitive function in Alzheimer Disease. Arch. Neurol. 1998, 55, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Lee, B.; Yun, B.R.; Lee, J.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. Memory enhancing effect of Codonopsis lanceolata by high hydrostatic pressure process and fermentation. Korean J. Pharmacogn. 2013, 44, 41–46. [Google Scholar]
- Hosseini, S.M.; Shahbazizadeh, S.; Khosravi-Darani, K.; Mozafari, M.R. Spirulina paltensis: Food and Function. Curr. Nutr. Food Sci. 2013, 9, 189–193. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell. Int. 2013, 13, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–783. [Google Scholar] [CrossRef] [PubMed]
- Son, M.H.; Park, K.H.; Choi, A.R.; Yoo, G.; In, M.J.; Kim, D.H.; Chae, H.J. Investigation of biological activities of enzymatic hydrolysate of Spirulina. J. Korean Soc. Food Sci. Nutr. 2009, 38, 136–141. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, E.H.; Kim, H.M. Spirulina platensis inhibits anaphylactic reaction. Life Sci. 1997, 61, 1237–1244. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.H.; Kim, J.S.; Han, J.A.; Seo, H.J.; Lim, H.J.; Choi, S.Y. Studies on simultaneous determination of chlorophyll a and b, pheophorbide a, and β-carotene in Chlorella and Spirulina products. J. Food Hyg. Saf. 2005, 20, 141–146. [Google Scholar]
- Yamada, T.; Sakaguchi, K. Comparative studies on Chlorella cell walls: Induction of protoplast formation. Arch. Microbiol. 1982, 132, 10–13. [Google Scholar] [CrossRef]
- Oh, S.H.; Ahn, J.H.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef]
- Eom, S.H.; Kang, Y.M.; Park, J.H.; Yu, D.U.; Jeong, E.T.; Lee, M.S.; Kim, Y.M. Enhancement of polyphenol content and antioxidant activity of brown alga Eisenia bicyclis extract by microbial fermentation. Fish. Aquat. Sci. 2011, 14, 192–197. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chan, C.F.; Huang, W.Y.; Lin, J.S.; Chan, P.; Liu, H.Y.; Lin, Y.S. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules 2013, 18, 14161–14171. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Lee, H.Y. Enhancement of Chlorophyll a Production from Marine Spirulina maxima by an Optimized Ultrasonic Extraction Process. Appl. Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Dietz, B.M.; Kang, Y.H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; Breemen, R.B.; et al. Xanthohumol isolated from humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef]
- Lee, J.; Weon, J.B.; Ma, C.J. Neuroprotective activity of phytosterols isolated from Artemisia apiacea. Korean J. Pharmacogn. 2014, 45, 214–219. [Google Scholar]
- Dar, M.A.; Khan, A.M.; Raina, R.; Beigh, S.A.; Sultana, M. Effect of repeated oral administration of bifenthrin antioxidant status and acetylcholinesterase activity in brain of rats. Toxicol. Environ. Chem. 2015, 97, 961–967. [Google Scholar] [CrossRef]
- Lee, M.R.; Moon, S.H.; Choi, A.R.; Lee, S.C.; Ahn, K.H.; Park, H.R. Neuroprotective effects of extracts from Diospyros kaki L. Peel. Korean J. Food Cookery Sci. 2011, 27, 67–73. [Google Scholar] [CrossRef]
- Kim, N.R.; Kim, H.Y.; Kim, M.H.; Kim, H.M.; Jeong, H.J. Improvement of depressive behavior by Sweetme Sweet Pumpkin™ and its active compound, β-carotene. Life Sci. 2016, 147, 39–45. [Google Scholar] [CrossRef]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Li, F.J.; Shen, L.; Ji, H.F. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Sergio, A.R.P.; Robert, M.R. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Kigoshi, M.; Iwatsuki, M.; Niki, E. Action of β-Carotene as an Antioxidant against Lipid Peroxidation. Arch. Biochem. Biophys. 1995, 323, 137–147. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. Vitamin E and β-carotene protect against ethanol combined with ischemia in an embryonic rat hippocampal culture model of fetal alcohol syndrome. Neurosci. Lett. 1999, 263, 189–192. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Paiva, M.; Heaton, M.B. The Antioxidants Vitamin E and β-Carotene Protect Against Ethanol-Induced Neurotoxicity in Embryonic Rat Hippocampal Cultures. Alcohol 1999, 17, 163–168. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Chen, D.Y.; Bambah-Mukku, D.; Pollonini, G.; Alberini, C.M. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat. Neurosci. 2012, 15, 1707–1714. [Google Scholar] [CrossRef] [Green Version]






| Extraction Process | β-Carotene Content (mg/g) | Extraction Yield (%) |
|---|---|---|
| FE 1 | 1.62 ± 0.22 | 18.26 ± 1.93 |
| WE 2 | 0.81 ± 0.13 | 10.60 ± 1.44 |
| UE 3 | 1.03 ± 0.34 | 12.77 ± 1.68 |
| Sample | Treated Concentration (µg/mL) | Glutathione (% of Control) | Glutathione Reductase (% of Control) | Glutathione Peroxidase (% of Control) |
|---|---|---|---|---|
| Control | - | 100.00 | 100.00 | 100.00 |
| Trolox | 50 μM | 91.88 ± 8.90 | 93.32 ± 7.21 | 91.26 ± 1.67 |
| Fermented Spirulina maxima extracts | 1 | 51.02 ± 3.31 | 61.42 ± 8.23 | 67.94 ± 5.50 |
| 10 | 63.76 ± 5.18 | 70.23 ± 4.49 | 75.07 ± 7.36 | |
| 100 | 80.38 ± 9.30 | 81.27 ± 5.11 | 80.33 ± 7.18 | |
| β-carotene | 1 | 47.73 ± 2.85 | 48.08 ± 1.07 | 43.75 ± 6.35 |
| Extraction Process | p-CREB/BDNF Expression Ratio |
|---|---|
| FE 1 | 1.338 |
| β-carotene | 1.055 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.Y.; Kang, D.H.; Heo, S.-J.; Lee, H.Y. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Appl. Sci. 2018, 8, 2469. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122469
Choi WY, Kang DH, Heo S-J, Lee HY. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Applied Sciences. 2018; 8(12):2469. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122469
Chicago/Turabian StyleChoi, Woon Yong, Do Hyung Kang, Soo-Jin Heo, and Hyeon Yong Lee. 2018. "Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction" Applied Sciences 8, no. 12: 2469. https://0-doi-org.brum.beds.ac.uk/10.3390/app8122469