Enhanced Harnessing of the Graviola Bioactive Components Using a Neoteric Sonication Cum Microwave Coadjuvant Extraction Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2 Characterization of Extracts
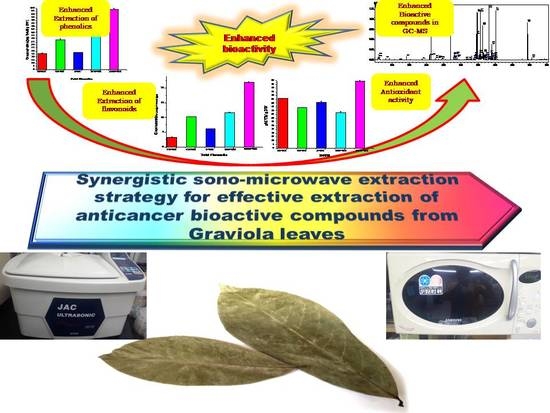
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflict of Interest
Ethical Approval
References
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the cancer killer): A review. Glob. J. Pharm. Res. 2013, 2, 1613–1618. [Google Scholar]
- De Souza, R.; Benassi, E.; da Silva, R.R.; Afonso, S.; Scarminio, I.S. Enhanced extraction yields and mobile phase separations by solvent mixtures for the analysis of metabolites in Annona muricata L. Leaves. J. Sep. Sci. 2009, 32, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Adewole, S.O.; Caxton-Martins, E.A. Morphological changes and hypoglycemic effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on pancreatic β-cells of streptozotocin-treated diabetic rats. Afr. J. Biomed. Res. 2006, 9, 173–187. [Google Scholar] [CrossRef]
- Leboeuf, M.; Cavé, A.; Bhaumik, P.K.; Mukherjee, B.; Mukherjee, R. The Phytochemistry of the Annonaceae. Phytochemistry 1980, 21, 2783–2813. [Google Scholar] [CrossRef]
- Taylor, L. Sage Bitter Melon (Momordica charantia). In Herbal Secrets of the Rainforest, 2nd ed.; Sage Press: Austin, TX, USA, 2002; Volume 10, pp. 1–6. [Google Scholar]
- Schultes, R.E.; Raffauf, R.F. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia; Dioscorides Press: Totnes, UK, 1992. [Google Scholar]
- Morton, J.F. Caribbean and Latin American folk medicine and its influence in the United States. Q. J. Crude Drug Res. 1980, 18, 57–75. [Google Scholar] [CrossRef]
- Leaman, D.J.; Arnason, J.T.; Yusuf, R.; Sangat-Roemantyo, H.; Soedjito, H.; Angerhofer, C.K.; Pezzuto, J.M. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: A quantitative assessment of local consensus as an indicator of biological efficacy. J. Ethnopharmacol. 1995, 49, 1–16. [Google Scholar] [CrossRef]
- Caribé, J. Plantas Que Ajudam o Homem: Guiaprático Para Aépocaatual; Cultrix/Pensamento: São Paulo, Brazil, 1999. [Google Scholar]
- Branch, L.C.; da Silva, M. Folk medicine of Alter does chao, para. Branza Acta Amazonica 1983, 13, 737–797. [Google Scholar] [CrossRef]
- Santos, A.F.D.; Sant’Ana, A.E.G. Molluscicidal Properties of Some Species of Annoa. Phytomedicine 2001, 8, 115–120. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, E. Plantas Medicinais Brasileiras: Conhecimentos Populares e Científicossao; Hemus: Sao Paulo, Brasil, 1993; Volume 4, ISBN 8528903095. [Google Scholar]
- Mors, W.B.; Rizzini, C.T.; Pereira, N.A. Medicinal Plants of Brazil; Reference Publications, Inc.: Algonac, MI, USA, 2000. [Google Scholar]
- Holdsworth, D.K. Traditional medicinal plants of Rarotonga, Cook Islands Part 1. Int. J. Crude Drug Res. 1990, 28, 209–218. [Google Scholar] [CrossRef]
- Morton, J.F. A survey of medicinal plants of Curacao. Econ. Bot. 1968, 22, 87–102. [Google Scholar] [CrossRef]
- Haddock, R.L. Report Regional Tech Mtg Med Plants; South Pacific Commission: Papeete, Tahiti, French Polynesia, 1973. [Google Scholar]
- Caceres, A.; Lopez, B.R.; Giron, M.A.; Logemann, H. Plants used in Guatemala for the treatment of dermatophytic infections: I. Screening for antimycotic activity of 44 plant extracts. J. Ethnopharmacol. 1991, 31, 263–276. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Chung, I.M.; Ahn, J.K.; Chi, H.Y.; Lee, J.O. Screening for antioxidative activity in soybean local cultivars in Korea. Korean J. Crop Sci. 2000, 45, 328–334. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharm. Biol. 2008, 43, 237–242. [Google Scholar] [CrossRef]
- Pandey, A.K. Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parihenium histerophorus: An in vitro study. Natl. Acad. Sci. Lett. 2007, 30, 383–386. [Google Scholar]
- Rice-Evans, C. Flavonoids and isoflavones: Absorption, metabolism and bioactivity. Free Radic. Biol. Med. 2004, 36, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.; Talcott, S.T.; Goodner, K.; Baldwin, E.A. Total antioxidant activity and fiber content of select florida-grown tropical fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.K.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.-F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; El-Shemy, H.A. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Dis. 2014, 7, S355–S363. [Google Scholar] [CrossRef]
- Paul, J.; Gnanam, R.; Jayadeepa, R.M.; Arul, L. Anti cancer activity on Graviola, an exciting medicinal plant extract vs. various cancer cell lines and a detailed computational study on its potent anti-cancerous leads. Curr. Top. Med. Chem. 2013, 13, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, J.; Kadouh, H.; Sun, X.; Zhou, K. Three new anti-proliferative Annonaceous acetogenins with mono-tetrahydrofuran ring from Graviola fruit (Annona muricata). Bioorg. Med. Chem. Lett. 2014, 24, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Thang, T.D.; Dai, D.N.; Hoi, T.M.; Ogunwande, I.A. Study on the volatile oil contents of Annona glabra L., Annona squamosa L., Annona muricata L. and Annona reticulata L., from Vietnam. Nat. Prod. Res. 2013, 27, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.; Ayoub, N.; Hussein, S.; Hashim, A.; El-Sharawy, R.; Wende, K.; Harms, M.; Lindequist, U. Flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata linn. Arch. Pharm. Res. 2012, 35, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a new aporphine alkaloid from the leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Matsushige, A.; Matsunami, K.; Kotake, Y.; Otsuka, H.; Ohta, S. Three new megastigmanes from the leaves of Annona muricata. J. Nat. Med. 2012, 66, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Guérineau, V.; Laprévote, O. MALDI-TOF MS profiling of Annonaceous acetogenins in Annona muricata products of human consumption. Molecules 2009, 14, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Pisano, P.L.; Silva, F.B.; Scaminio, I.S.; Olivieri, A.C. Discrimination of Annona muricata and Rollinia mucosa Extracts by Using Multivariate Curve Resolution and Partial Least-Squares Regression of Liquid Chromatography-Diode Array Data. J. Braz. Chem. Soc. 2015, 26, 2241–2248. [Google Scholar]
- Rupprecht, J.K.; Hui, Y.-H.; McLaughlin, J.L. Annonaceous acetogenins: A review. J. Nat. Prod. 1990, 53, 237–278. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.; Kung, F.W. Microwave-assisted extraction of active ingredients from plants—A review. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Bandar, H.; Hijazi, A.; Rammal, H.; Hachem, A.; Saad, Z.; Badran, B. Techniques for the Extraction of Bioactive Compounds from Lebanese Urticadioica. Am. J. Phytomed. Clin. Ther. 2013, 6, 507–513. [Google Scholar]
- Chemat, F.; Abert-Vian, M.; Zill-e-Huma, Y.-J. Microwave assisted separations: Green chemistry in action. In Green Chemistry Research Trends; Pearlman, J.T., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 33–62. [Google Scholar]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of ultrasonically assisted extraction of bioactive herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, S.C.; Xiaomin, S.; Anthonydhason, V.; Jung, H.; Tilahun Belachew, S.; Gopal, J.; Paul, D. Enhanced Harnessing of the Graviola Bioactive Components Using a Neoteric Sonication Cum Microwave Coadjuvant Extraction Protocol. Appl. Sci. 2018, 8, 232. https://0-doi-org.brum.beds.ac.uk/10.3390/app8020232
Chun SC, Xiaomin S, Anthonydhason V, Jung H, Tilahun Belachew S, Gopal J, Paul D. Enhanced Harnessing of the Graviola Bioactive Components Using a Neoteric Sonication Cum Microwave Coadjuvant Extraction Protocol. Applied Sciences. 2018; 8(2):232. https://0-doi-org.brum.beds.ac.uk/10.3390/app8020232
Chicago/Turabian StyleChun, Se Chul, Shang Xiaomin, Vimala Anthonydhason, Hyejin Jung, Shimels Tilahun Belachew, Judy Gopal, and Diby Paul. 2018. "Enhanced Harnessing of the Graviola Bioactive Components Using a Neoteric Sonication Cum Microwave Coadjuvant Extraction Protocol" Applied Sciences 8, no. 2: 232. https://0-doi-org.brum.beds.ac.uk/10.3390/app8020232