A Review of the Synthesis and Applications of Polymer–Nanoclay Composites
Abstract
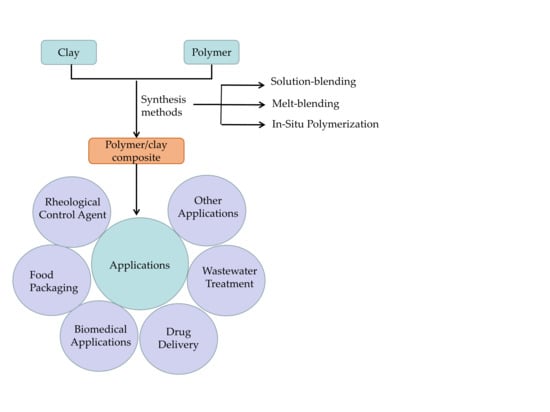
:1. Introduction
2. Synthesis
2.1. Solution-Blending Method
2.2. Melt-Blending Method
2.3. In-Situ Polymerization Method
2.3.1. Surface-Initiated Controlled/Living Radical Polymerization (SI-CLRP)
2.3.2. Controlled Radical-Mediated Photopolymerization (P-CRP)
2.3.3. Click Coupling Chemistry
2.3.4. Miniemulsion Polymerization
3. Application
3.1. Rheological Control Agent
3.2. Food Packaging
3.3. Biomedical Applications and Drug Delivery
3.4. Wastewater Treatment
3.5. Other Applications
4. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazir, M.S.; Kassim, M.H.M.; Mohapatra, L.; Gilani, M.A.; Raza, M.R.; Majeed, K. Characteristic properties of nanoclays and characterization of nanoparticulates and nanocomposites. In Nanoclay Reinforced Polymer Composites; Springer: Singapore, 2016; pp. 35–55. [Google Scholar]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G.J.M. Clay minerals as an ancient nanotechnology: Historical uses of clay organic interactions, and future possible perspectives. Macla 2008, 9, 15–17. [Google Scholar]
- Lee, S.M.; Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59, 84–102. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Jawaid, M.; Qaiss, A.K.; Bouhfid, R. Nanoclay Reinforced Polymer Composites: Nanocomposites and Bionanocomposites; Springer: Singapore, 2016. [Google Scholar]
- Savic, I.; Stojiljkovic, S.; Savic, I.; Gajic, D. Industrial application of clays and clay minerals. In Clays and Clay Minerals: Geological Origin, Mechanical Properties and Industrial Applications; Wesley, L.R., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 379–402. [Google Scholar]
- Barton, C.D.; Karathanasis, A.D. Clay minerals. In Encyclopedia of Soil Science; Lal, R., Ed.; Taylor & Francis: Guelph, ON, Canada, 2016; p. 276. [Google Scholar]
- Majeed, K.; Jawaid, M.; Hassan, A.; Abu Bakar, A.; Abdul Khalil, H.P.S.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Deng, H.; Bai, H.; Zhang, Q.; Wang, K.; Chen, F.; Fu, Q. Confine clay in an alternating multilayered structure through injection molding: A simple and efficient route to improve barrier performance of polymeric materials. ACS Appl. Mater. Interfaces 2015, 7, 10178–10189. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.B.; Gilman, J. Polymer-clay nanocomposites: Design and application of multi-functional materials. Mater. Matters 2007, 2, 20–25. [Google Scholar]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Yusoh, K.; Kumaran, S.V.; Ismail, F.S. Surface Modification of Nanoclay for the Synthesis of Polycaprolactone (PCL)—Clay Nanocomposite. In Proceedings of the MATEC Web of Conferences, Penang, Malaysia, 6–7 December 2017. [Google Scholar]
- Irshidat, M.R.; Al-Saleh, M.H. Thermal performance and fire resistance of nanoclay modified cementitious materials. Constr. Build. Mater. 2018, 159, 213–219. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Ganguly, S.; Dana, K.; Mukhopadhyay, T.K.; Parya, T.; Ghatak, S. Organophilic nano clay: A Comprehensive review. Trans. Indian Ceram. Soc. 2011, 70, 189–206. [Google Scholar] [CrossRef]
- Öztürk, N.; Tabak, A.; Akgöl, S.; Denizli, A. Newly synthesized bentonite–histidine (Bent–His) micro-composite affinity sorbents for IgG adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 490–497. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Kadhum, A.; Al-Amiery, A.; Nassir, M.; Jaaz, A. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Ambre, A.H.; Katti, K.S.; Katti, D.R. Nanoclay Based Composite Scaffolds for Bone Tissue Engineering Applications. J. Nanotechnol. Eng. Med. 2010, 1, 031013. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Shahidi, S.; Ghoranneviss, M. Effect of Plasma Pretreatment Followed by Nanoclay Loading on Flame Retardant Properties of Cotton Fabric. J. Fusion Energy 2014, 33, 88–95. [Google Scholar] [CrossRef]
- Cudjoe, E.; Khani, S.; Way, A.E.; Hore, M.J.A.; Maia, J.; Rowan, S.J. Biomimetic Reversible Heat-Stiffening Polymer Nanocomposites. ACS Cent. Sci. 2017, 3, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Interlayer polymerization in amine-terminated macromolecular chain-grafted expanded graphite for fabricating highly thermal conductive and physically strong thermoset composites for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2018, 109, 498–506. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Kausar, A.; Muhammad, B.; Shah, S. Progression from Graphene and Graphene Oxide to High Performance Polymer-Based Nanocomposite: A Review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. In situ shear-induced mercapto group-activated graphite nanoplatelets for fabricating mechanically strong and thermally conductive elastomer composites for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2018, 112, 40–48. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Abdelraheem, A.; El-Shazly, A.; Elkady, M. Synthesis and Characterization of Intercalated Polyaniline-Clay Nanocomposite Using Supercritical CO2. AIP Conf. Proc. 1968, 020027. [Google Scholar] [CrossRef]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; iSmithers Rapra Publishing: Shawbury, UK, 2004; Volume 1. [Google Scholar]
- Ozkose, U.U.; Altinkok, C.; Yilmaz, O.; Alpturk, O.; Tasdelen, M.A. In-situ preparation of poly(2-ethyl-2-oxazoline)/clay nanocomposites via living cationic ring-opening polymerization. Eur. Polym. J. 2017, 88, 586–593. [Google Scholar] [CrossRef]
- Karamane, M.; Raihane, M.; Tasdelen, M.A.; Uyar, T.; Lahcini, M.; Ilsouk, M.; Yagci, Y. Preparation of fluorinated methacrylate/clay nanocomposite via in-situ polymerization: Characterization, structure, and properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 411–418. [Google Scholar] [CrossRef]
- Saad, A.; Jlassi, K.; Omastová, M.; Chehimi, M.M. Clay/Conductive Polymer Nanocomposites. In Clay-Polymer Nanocomposites; Elsevier: Cambridge, MA, USA, 2017; pp. 199–237. [Google Scholar]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly (GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.S.; Mahouche-Chergui, S.; Babinot, J.; Nguyen, V.H.; Naili, S.; Carbonnier, B. Photo-induced SI-ATRP for the synthesis of photoclickable intercalated clay nanofillers. RSC Adv. 2016, 6, 89322–89327. [Google Scholar] [CrossRef]
- Guerrouache, M.; Mahouche-Chergui, S.; Chehimi, M.M.; Carbonnier, B. Site-specific immobilisation of gold nanoparticles on a porous monolith surface by using a thiol-yne click photopatterning approach. Chem. Commun. 2012, 48, 7486–7488. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, V.; Kokotidou, C.; Le Droumaguet, B.; Carbonnier, B.; Choli-Papadopoulou, T.; Dendrinou-Samara, C. Oleylamine as a beneficial agent for the synthesis of CoFe2O4 nanoparticles with potential biomedical uses. Dalton Trans. 2014, 43, 6377–6388. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Synthesis and Characterization of PMMA/Organomodified Montmorillonite Nanocomposites Prepared by in Situ Bulk Polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Atilla Tasdelen, M.; Yagci, Y. Photoinitiated atom transfer radical polymerization: Current status and future perspectives. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2878–2888. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, A.; Zhang, L.; Cavicchi, K.A.; Weiss, R.A.; Singha, N.K. Tailor-Made Fluorinated Copolymer/Clay Nanocomposite by Cationic RAFT Assisted Pickering Miniemulsion Polymerization. Langmuir 2015, 31, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Atilla, T.M.; Johannes, K.; Yusuf, Y. In situ Synthesis of Polymer/Clay Nanocomposites by Living and Controlled/Living Polymerization. Macromol. Chem. Phys. 2010, 211, 279–285. [Google Scholar] [CrossRef]
- Schmidt, D.; Shah, D.; Giannelis, E.P. New advances in polymer/layered silicate nanocomposites. Curr. Opin. Solid State Mater. Sci. 2002, 6, 205–212. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Gacitua, W.; Ballerini, A.; Zhang, J. Polymer nanocomposites: Synthetic and natural fillers a review. Maderas. Cienc. Tecnol. 2005, 7, 159–178. [Google Scholar] [CrossRef]
- Vo, V.-S.; Mahouche-Chergui, S.; Nguyen, V.-H.; Naili, S.; Singha, N.K.; Carbonnier, B. Chemical and Photochemical Routes Toward Tailor-Made Polymer–Clay Nanocomposites: Recent Progress and Future Prospects. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–197. [Google Scholar]
- Jlassi, K.; Krupa, I.; Chehimi, M.M. Overview: Clay Preparation, Properties, Modification. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–28. [Google Scholar]
- Babu Valapa, R.; Loganathan, S.; Pugazhenthi, G.; Thomas, S.; Varghese, T.O. An Overview of Polymer–Clay Nanocomposites. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–81. [Google Scholar]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Beyer, G. Nanocomposites: A new class of flame retardants for polymers. Plast. Addit. Compd. 2002, 4, 22–28. [Google Scholar] [CrossRef]
- Gurses, A. Introduction to Polymer-Clay Nanocomposites; Pan Stanford: Singapore, 2015. [Google Scholar]
- Fischer, H.; Gielgens, L.; Koster, T. Nanocomposites from polymers and layered minerals. Acta Polym. 1999, 50, 122–126. [Google Scholar] [CrossRef]
- Mittal, V. Advances in Polyolefin Nanocomposites; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Patil, S.S.; Thomas, S. Clay–Polymer Composites: Design of Clay Polymer Nanocomposite by Mixing. In Clay-Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–144. [Google Scholar]
- Buruga, K.; Kalathi, J.T. A facile synthesis of halloysite nanotubes based polymer nanocomposites for glass coating application. J. Alloys Compd. 2018, 735, 1807–1817. [Google Scholar] [CrossRef]
- Lopez-Manchado, M.; Herrero, B.; Arroyo, M. Organoclay–natural rubber nanocomposites synthesized by mechanical and solution mixing methods. Polym. Int. 2004, 53, 1766–1772. [Google Scholar] [CrossRef]
- Maiti, M.; Sadhu, S.; Bhowmick, A.K. Ethylene–octene copolymer (engage)–clay nanocomposites: Preparation and characterization. J. Appl. Polym. Sci. 2006, 101, 603–610. [Google Scholar] [CrossRef]
- Hausner, J.; Ziadeh, M.; Fischer, B.; Kalo, H.; Schmid, J.; Kunz, R.; Altstädt, V.; Breu, J. Transfer batch blending, an innovative solvent/solid assisted method for melt compounding to achieve good dispersion quality for polymer–clay-nanocomposites. Compos. Sci. Technol. 2015, 114, 34–41. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Microporous Mesoporous Mater. 2016, 232, 273–280. [Google Scholar] [CrossRef]
- He, F.; Lam, K.-H.; Fan, J.; Chan, L.H. Improved dielectric properties for chemically functionalized exfoliated graphite nanoplates/syndiotactic polystyrene composites prepared by a solution-blending method. Carbon 2014, 80, 496–503. [Google Scholar] [CrossRef]
- Lago, E.; Toth, P.S.; Pugliese, G.; Pellegrini, V.; Bonaccorso, F. Solution blending preparation of polycarbonate/graphene composite: Boosting the mechanical and electrical properties. RSC Adv. 2016, 6, 97931–97940. [Google Scholar] [CrossRef]
- Abbasian, M.; Pakzad, M.; Amirmanesh, M. Polymericaly modified clays to preparation of polystyrene nanocomposite by nitroxide mediated radical polymerization and solution blending methods. Polym. Compos. 2017, 38, 1127–1134. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Çiftçi, H. Synthesis and characterization of chitosan/ polyvinylpyrrolidone/ zeolite composite by solution blending method. J. Inorg. Organomet. Polym. Mater. 2014, 24, 1048–1054. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Krishna, S.; Pugazhenthi, G. Structural and thermal properties of polystyrene/CoAl-layered double hydroxide nanocomposites prepared via solvent blending: Effect of LDH loading. J. Exp. Nanosci. 2013, 8, 19–31. [Google Scholar] [CrossRef]
- Nanda, R.; Sasmal, A.; Nayak, P. Preparation and characterization of chitosan–polylactide composites blended with Cloisite 30B for control release of the anticancer drug paclitaxel. Carbohydr. Polym. 2011, 83, 988–994. [Google Scholar] [CrossRef]
- Becker, C.M.; Gabbardo, A.D.; Wypych, F.; Amico, S.C. Mechanical and flame-retardant properties of epoxy/Mg–Al LDH composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 196–202. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Shi, W. A novel approach for preparing exfoliated UV-cured polymer/LDH nanocomposites via pre-exfoliated organic LDH. Appl. Clay Sci. 2011, 53, 608–614. [Google Scholar] [CrossRef]
- Choudhury, A.; Bhowmick, A.K.; Ong, C.; Soddemann, M. Effect of various nanofillers on thermal stability and degradation kinetics of polymer nanocomposites. J. Nanosci. Nanotechnol. 2010, 10, 5056–5071. [Google Scholar] [CrossRef] [PubMed]
- Debnath, D.; Dhibar, A.K.; Khatua, B. Studies on the morphology and properties of PMMA-organoclay nanocomposites with reference to the manufacturing techniques. Polym. Plast. Technol. Eng. 2010, 49, 1087–1094. [Google Scholar] [CrossRef]
- Ports, B.F.; Weiss, R. One-step melt extrusion process for preparing polyolefin/clay nanocomposites using natural montmorillonite. Ind. Eng. Chem. Res. 2010, 49, 11896–11905. [Google Scholar] [CrossRef]
- Jollands, M.; Gupta, R.K. Effect of mixing conditions on mechanical properties of polylactide/montmorillonite clay nanocomposites. J. Appl. Polym. Sci. 2010, 118, 1489–1493. [Google Scholar] [CrossRef]
- Yarahmadi, N.; Jakubowicz, I.; Hjertberg, T. Development of poly(vinyl chloride)/montmorillonite nanocomposites using chelating agents. Polym. Degrad. Stab. 2010, 95, 132–137. [Google Scholar] [CrossRef]
- Albdiry, M.; Yousif, B.; Ku, H.; Lau, K. A critical review on the manufacturing processes in relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2013, 47, 1093–1115. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Ge, T.; Kalathi, J.T.; Halverson, J.D.; Grest, G.S.; Rubinstein, M. Nanoparticle Motion in Entangled Melts of Linear and Nonconcatenated Ring Polymers. Macromolecules 2017, 50, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Mahdis, H.; Azam, J.-A. Morphology Development via Static Crosslinking of (Polylactic Acid/Acrylic Rubber) as an Immiscible Polymer Blend. Macromol. Mater. Eng. 2018, 303, 1700446. [Google Scholar] [CrossRef]
- Coativy, G.; Misra, M.; Mohanty, A.K. Microwave Synthesis and Melt Blending of Glycerol Based Toughening Agent with Poly(lactic acid). ACS Sustain. Chem. Eng. 2016, 4, 2142–2149. [Google Scholar] [CrossRef]
- Martin, C. Twin Screw Extruders as Continuous Mixers for Thermal Processing: A Technical and Historical Perspective. AAPS PharmSciTech 2016, 17, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.M.; Viana, J.C. Melt blending and characterization of carbon nanoparticles-filled thermoplastic polyurethane elastomers. J. Elastomers Plast. 2015, 47, 647–665. [Google Scholar] [CrossRef] [Green Version]
- Burmistr, M.V.; Sukhyy, K.M.; Shilov, V.V.; Pissis, P.; Spanoudaki, A.; Sukha, I.V.; Tomilo, V.I.; Gomza, Y.P. Synthesis, structure, thermal and mechanical properties of nanocomposites based on linear polymers and layered silicates modified by polymeric quaternary ammonium salts (ionenes). Polymer 2005, 46, 12226–12232. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.; Mamat, M.; Bee, S.-T.; Sin, L.T.; Hui, D.; Rahmat, A. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part B Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Beuguel, Q.; Ville, J.; Crepin-Leblond, J.; Mederic, P.; Aubry, T. Influence of clay mineral structure and polyamide polarity on the structural and morphological properties of clay polypropylene/polyamide nanocomposites. Appl. Clay Sci. 2017, 135, 253–259. [Google Scholar] [CrossRef]
- Moustafa, H.; Galliard, H.; Vidal, L.; Dufresne, A. Facile modification of organoclay and its effect on the compatibility and properties of novel biodegradable PBE/PBAT nanocomposites. Eur. Polym. J. 2017, 87, 188–199. [Google Scholar] [CrossRef]
- Vassiljeva, V.; Kirikal, K.-K.; Hietala, S.; Kaljuvee, T.; Mikli, V.; Rähn, M.; Tarasova, E.; Krasnou, I.; Viirsalu, M.; Savest, N. One-step carbon nanotubes grafting with styrene-co-acrylonitrile by reactive melt blending for electrospinning of conductive reinforced composite membranes. Fullerenes Nanotub. Carbon Nanostruct. 2017, 25, 667–677. [Google Scholar] [CrossRef]
- Ercan, N.; Durmus, A.; Kaşgöz, A. Comparing of melt blending and solution mixing methods on the physical properties of thermoplastic polyurethane/organoclay nanocomposite films. J. Thermoplast. Compos. Mater. 2017, 30, 950–970. [Google Scholar] [CrossRef]
- Quigley, J.P.; Baird, D.G. Improved mechanical properties of organoclay/nylon 6 nanocomposites prepared via a supercritical carbon dioxide-aided, melt blending method. Polym. Compos. 2015, 36, 527–537. [Google Scholar] [CrossRef]
- Liang, C.; Hu, C.; Zheng, Y.; Yan, K.; Zhu, X. Modification of isotactic polypropylene by silica nanocapsules via melt blending method. Polym. Compos. 2018, 39, 762–769. [Google Scholar] [CrossRef]
- Yilmaz, B.; Doğan, S.; Çelikler Kasimoğullari, S. Hemocompatibility, cytotoxicity, and genotoxicity of poly(methylmethacrylate)/nanohydroxyapatite nanocomposites synthesized by melt blending method. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 351–360. [Google Scholar] [CrossRef]
- Vasudeo, R.A.; Samarth, N.; Jadhav, S.; Patil, S.; Narute, S. Development in air permeability of natural rubber tire tube compound by adding variable dosage of nanoclay. Macromol. Sym. 2016, 360, 34–41. [Google Scholar] [CrossRef]
- Paci, M.; Filippi, S.; Magagnini, P. Nanostructure development in nylon 6-Cloisite® 30B composites. Effects of the preparation conditions. Eur. Polym. J. 2010, 46, 838–853. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Clay network in ABS-graft-MAH nanocomposites: Rheology and flammability. Polym. Degrad. Stab. 2007, 92, 1439–1445. [Google Scholar] [CrossRef]
- Boukerrou, A.; Duchet, J.; Fellahi, S.; Kaci, M.; Sautereau, H. Morphology and mechanical and viscoelastic properties of rubbery epoxy/organoclay montmorillonite nanocomposites. J. Appl. Polym. Sci. 2007, 103, 3547–3552. [Google Scholar] [CrossRef]
- Han, B.; Ji, G.; Wu, S.; Shen, J. Preparation and characterization of nylon 66/montmorillonite nanocomposites with co-treated montmorillonites. Eur. Polym. J. 2003, 39, 1641–1646. [Google Scholar] [CrossRef]
- Raka, L.; Bogoeva-Gaceva, G.; Lu, K.; Loos, J. Characterization of latex-based isotactic polypropylene/clay nanocomposites. Polymer 2009, 50, 3739–3746. [Google Scholar] [CrossRef]
- Matuana, L.M. Rigid PVC/(layered silicate) nanocomposites produced through a novel melt-blending approach. J. Vinyl Addit. Technol. 2009, 15, 77–86. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, K.; Dong, J.Y. Copolymerization of Ethylene and 10-Undecen-1-ol using a Montmorillonite-Intercalated Metallocene Catalyst: Synthesis of Polyethylene/Montmorillonite Nanocomposites with Enhanced Structural Stability. Macromol. Rapid Commun. 2006, 27, 1278–1283. [Google Scholar] [CrossRef]
- Abedi, S.; Abdouss, M. A review of clay-supported Ziegler–Natta catalysts for production of polyolefin/clay nanocomposites through in situ polymerization. Appl. Catal. A Gen. 2014, 475, 386–409. [Google Scholar] [CrossRef]
- Asensio, M.; Herrero, M.; Núñez, K.; Gallego, R.; Merino, J.C.; Pastor, J.M. In situ polymerization of isotactic polypropylene sepiolite nanocomposites and its copolymers by metallocene catalysis. Eur. Polym. J. 2018, 100, 278–289. [Google Scholar] [CrossRef]
- Salmi, Z.; Benzarti, K.; Chehimi, M.M. Diazonium Cation-Exchanged Clay: An Efficient, Unfrequented Route for Making Clay/Polymer Nanocomposites. Langmuir 2013, 29, 13323–13328. [Google Scholar] [CrossRef] [PubMed]
- Alicia, C.; Rafael, V.G.; Inmaculada, S.; Beatriz, P. Development of a new synthetic method based on in situ strategies for polyethylene/clay composites. J. Appl. Polym. Sci. 2012, 126, 987–997. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Yang, C.; Sakai, E. Preparation and application of conducting polymer/Ag/clay composite nanoparticles formed by in situ UV-induced dispersion polymerization. Sci. Rep. 2016, 6, 20470. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Núñez, K.; Gallego, R.; Merino, J.C.; Pastor, J.M. Control of molecular weight and polydispersity in polyethylene/needle-like shaped clay nanocomposites obtained by in situ polymerization with metallocene catalysts. Eur. Polym. J. 2016, 75, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Kherroub, D.E.; Belbachir, M.; Lamouri, S. Synthesis of poly(furfuryl alcohol)/montmorillonite nanocomposites by direct in-situ polymerization. Bull. Mater. Sci. 2015, 38, 57–63. [Google Scholar] [CrossRef]
- Prado, B.R.; Bartoli, J.R. Synthesis and characterization of PMMA and organic modified montmorilonites nanocomposites via in situ polymerization assisted by sonication. Appl. Clay Sci. 2018, 160, 132–143. [Google Scholar] [CrossRef]
- Sharma, S.; Poddar, M.K.; Moholkar, V.S. Enhancement of thermal and mechanical properties of poly(MMA-co-BA)/Cloisite 30B nanocomposites by ultrasound-assisted in-situ emulsion polymerization. Ultrason. Sonochem. 2017, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.S.; Aguiar, V.O.; Marques, M.D.F.V. Masterbatches of polypropylene/clay obtained by in situ polymerization and melt-blended with commercial polypropylene. J. Compos. Mater. 2017, 51, 3547–3556. [Google Scholar] [CrossRef]
- Hua, J.; Liu, J.; Wang, X.; Yue, Z.; Yang, H.; Geng, J.; Ding, A. Structure and properties of a cis-1, 4-polybutadiene/organic montmorillonite nanocomposite prepared via in situ polymerization. J. Macromol. Sci. Part B 2017, 56, 451–461. [Google Scholar] [CrossRef]
- Boukoussa, B.; Abidallah, F.; Abid, Z.; Talha, Z.; Taybi, N.; El Hadj, H.S.; Ghezini, R.; Hamacha, R.; Bengueddach, A. Synthesis of polypyrrole/Fe-kanemite nanocomposite through in situ polymerization: Effect of iron exchange, acid treatment, and CO2 adsorption properties. J. Mater. Sci. 2017, 52, 2460–2472. [Google Scholar] [CrossRef]
- Colonna, M.; Acquasanta, F.; Gioia, C.; Celli, A. Effect of telechelic ionic groups on the dispersion of organically modified clays in bisphenol A polycarbonate nanocomposites by in-situ polymerization using activated carbonates. Express Polym. Lett. 2017, 11, 396. [Google Scholar] [CrossRef]
- Nair, P.P.; George, K.; Jayakrishnan, N. Studies on mechanical behavior high impact polystyrene/vinyl clay nanocomposites: Comparison between in situ polymerization and melt mixing. Polym. Compos. 2017, 38, 68–76. [Google Scholar] [CrossRef]
- Marques, M.D.F.V.; Fernandes, R.M. Influence of Polypropylene Reaction Time on the Clay Exfoliation Process by In Situ Polymerization. J. Nanosci. Nanotechnol. 2017, 17, 5095–5103. [Google Scholar] [CrossRef]
- Hossein, R.-M.; Vahid, H.-A.; Mohammad, N.; Mehdi, S.-K. Synthesis and characterization of clay dispersed polystyrene nanocomposite via atom transfer radical polymerization. Polym. Compos. 2010, 31, 1829–1837. [Google Scholar] [CrossRef]
- Lee, K.M.; Han, C.D. Linear Dynamic Viscoelastic Properties of Functionalized Block Copolymer/Organoclay Nanocomposites. Macromolecules 2003, 36, 804–815. [Google Scholar] [CrossRef]
- Konn, C.; Morel, F.; Beyou, E.; Chaumont, P.; Bourgeat-Lami, E. Nitroxide-Mediated Polymerization of Styrene Initiated from the Surface of Laponite Clay Platelets. Macromolecules 2007, 40, 7464–7472. [Google Scholar] [CrossRef]
- Ambade, A.V. Controlled radical polymerization. In Metal-Catalyzed Polymerization; CRC Press: Boca Raton, FL, USA, 2017; pp. 161–177. [Google Scholar]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.B.; Bourgeat-Lami, E.; Lansalot, M. Controlled/living radical polymerization in dispersed systems: An update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.M.; Pietrasik, J.; Schmitt, M.; Mahoney, C.; Choi, J.; Bockstaller, M.R.; Matyjaszewski, K. Surface-Initiated Polymerization as an Enabling Tool for Multifunctional (Nano-)Engineered Hybrid Materials. Chem. Mater. 2014, 26, 745–762. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, J.; Li, K.; Zhang, Y.; Liu, J. Grafting Amphiphilic Brushes onto Halloysite Nanotubes via a Living RAFT Polymerization and Their Pickering Emulsification Behavior. J. Phys. Chem. B 2014, 118, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Le-Masurier, S.; Gody, G.; Perrier, S.; Granville, A. One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and “grafting from” RAFT polymerization. Polym. Chem. 2014, 5, 2816–2823. [Google Scholar] [CrossRef]
- Utama, R.H.; Drechsler, M.; Förster, S.; Zetterlund, P.B.; Stenzel, M.H. Synthesis of pH-responsive nanocapsules via inverse miniemulsion periphery RAFT polymerization and post-polymerization reaction. ACS Macro Lett. 2014, 3, 935–939. [Google Scholar] [CrossRef]
- Nikolaidis, A.; Achilias, D. Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization. Polymers 2018, 10, 491. [Google Scholar] [CrossRef]
- Beyazit, S.; Bui, B.T.S.; Haupt, K.; Gonzato, C. Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci. 2016, 62, 1–21. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef] [Green Version]
- Tehfe, M.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef] [Green Version]
- Lorandi, F.; Fantin, M.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. New protocol to determine the equilibrium constant of atom transfer radical polymerization. Electrochim. Acta 2018, 260, 648–655. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, K.; Chandran, S.; Mičušik, M.; Benna-Zayani, M.; Yagci, Y.; Thomas, S.; Chehimi, M.M. Poly(glycidyl methacrylate)-grafted clay nanofiller for highly transparent and mechanically robust epoxy composites. Eur. Polym. J. 2015, 72, 89–101. [Google Scholar] [CrossRef]
- Jlassi, K.; Benna-Zayani, M.; Chehimi, M.M.; Yagci, Y. Efficient photoinduced In situ preparation of clay/poly(glycidyl methacrylate) nanocomposites using hydrogen-donor silane. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 800–808. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Q.; Shi, W. Preparation of photopolymerized nanocomposites through intercalating multifunctional acrylated siloxane into montmorillonite. Appl. Clay Sci. 2014, 99, 164–170. [Google Scholar] [CrossRef]
- Ohtsuki, A.; Lei, L.; Tanishima, M.; Goto, A.; Kaji, H. Photocontrolled organocatalyzed living radical polymerization feasible over a wide range of wavelengths. J. Am. Chem. Soc. 2015, 137, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Boyer, C. Stereo-, temporal and chemical control through photoactivation of living radical polymerization: Synthesis of block and gradient copolymers. J. Am. Chem. Soc. 2015, 137, 9988–9999. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Johnson, J.A. Improving photo-controlled living radical polymerization from trithiocarbonates through the use of continuous-flow techniques. Chem. Commun. 2015, 51, 6742–6745. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Tasdelen, M. Polymer Nanocomposites via Click Chemistry Reactions. Polymers 2017, 9, 499. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Wang, Y.; Zhang, W. Recent advances in organic–inorganic well-defined hybrid polymers using controlled living radical polymerization techniques. Polym. Chem. 2016, 7, 3950–3976. [Google Scholar] [CrossRef]
- Yadav, P.; Chacko, S.; Kumar, G.; Ramapanicker, R.; Verma, V. Click chemistry route to covalently link cellulose and clay. Cellulose 2015, 22, 1615–1624. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Wu, Y.; Song, H.; Ba, X. High-efficiency grafting of halloysite nanotubes by using π-conjugated polyfluorenes via “click” chemistry. J. Mater. Sci. 2015, 50, 4387–4395. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, P. Surface Modification of Attapulgite Nanorods with Nitrile Butadiene Rubber via Thiol–Ene Interfacial Click Reaction: Grafting or Crosslinking. Ind. Eng. Chem. Res. 2018, 57, 4949–4954. [Google Scholar] [CrossRef]
- Ballard, N.; Salsamendi, M.; Carretero, P.; Asua, J.M. An investigation into the nature and potential of in-situ surfactants for low energy miniemulsification. J. Colloid Interface Sci. 2015, 458, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yao, H.; Ma, J. Recent advances in RAFT-mediated surfactant-free emulsion polymerization. Polym. Chem. 2018, 9, 2532–2561. [Google Scholar] [CrossRef]
- Wang, Y.; Dadashi-Silab, S.; Matyjaszewski, K. Photoinduced Miniemulsion Atom Transfer Radical Polymerization. ACS Macro Lett. 2018, 7, 720–725. [Google Scholar] [CrossRef]
- Chanra, J.; Budianto, E.; Soegijono, B. Synthesis of polymer hybrid latex poly(methyl methacrylate-co-butyl acrylate) with organo montmorillonite via miniemulsion polymerization method for barrier paper. J. Phys. Conf. Ser. 2018, 985, 012029. [Google Scholar] [CrossRef]
- Buruga, K.; Kalathi, J.T. Fabrication of γ-MPS-Modified HNT–PMMA Nanocomposites by Ultrasound-Assisted Miniemulsion Polymerization. JOM 2018, 70, 1307–1312. [Google Scholar] [CrossRef]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research progress on properties and applications of polymer/clay nanocomposite. Polym. Plast. Technol. Eng. 2016, 55, 684–703. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog. Polym. Sci. 2018, 80, 1–38. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications; Elsevier: Amsteram, The Netherlands, 2017. [Google Scholar]
- Yang, J.; Tighe, S. A review of advances of nanotechnology in asphalt mixtures. Procedia Soc. Behav. Sci. 2013, 96, 1269–1276. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Pan, D.; Vipulanandan, C.; Amani, N.; Reddy, S.A.; Chockalingam, C.G. Effects of Nanoclay on the Rheological Properties and Resistivity of Synthetic Based Drilling Fluids under High Temperature. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2018. [Google Scholar]
- Dong, Q.; Yuan, J.; Chen, X.; Ma, X. Reduction of moisture susceptibility of cold asphalt mixture with Portland cement and bentonite nanoclay additives. J. Clean. Prod. 2018, 176, 320–328. [Google Scholar] [CrossRef]
- You, Z.; Mills-Beale, J.; Foley, J.M.; Roy, S.; Odegard, G.M.; Dai, Q.; Goh, S.W. Nanoclay-modified asphalt materials: Preparation and characterization. Constr. Build. Mater. 2011, 25, 1072–1078. [Google Scholar] [CrossRef]
- Rutherford, T.; Wang, Z.; Shu, X.; Huang, B.; Clarke, D. Laboratory investigation into mechanical properties of cement emulsified asphalt mortar. Constr. Build. Mater. 2014, 65, 76–83. [Google Scholar] [CrossRef]
- Merritt, S.; Wan, C.; Shollock, B.; Patole, S.; Haddleton, D.M. Polymer/Graphene Nanocomposites for Food Packaging. Compos. Mater. Food Packag. 2018, 251–267. [Google Scholar]
- Perchonok, M. NASA, We Have a Challenge and It’s Food Packaging. Presented at 2014 Institute of Food Technologists Annual Meeting, New Orleans, LA, USA, 21–24 June 2014. [Google Scholar]
- Yahiaoui, F.; Benhacine, F.; Ferfera-Harrar, H.; Habi, A.; Hadj-Hamou, A.S.; Grohens, Y. Development of antimicrobial PCL/nanoclay nanocomposite films with enhanced mechanical and water vapor barrier properties for packaging applications. Polym. Bull. 2015, 72, 235–254. [Google Scholar]
- Savas, L.A.; Hancer, M. Montmorillonite reinforced polymer nanocomposite antibacterial film. Appl. Clay Sci. 2015, 108, 40–44. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S.; Najafi, A. Preparation and characterization of bionanocomposite films reinforced with nano kaolin. J. Food Sci. Technol. 2016, 53, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Murima, D.; Pfukwa, H.; Tiggelman, I.; Hartmann, P.C.; Pasch, H. Novel Polymer Clay-Based Nanocomposites: Films with Remarkable Optical and Water Vapor Barrier Properties. Macromol. Mater. Eng. 2016, 301, 836–845. [Google Scholar] [CrossRef]
- Behroozi, M.; Pakizeh, M. Study the effects of C loisite15 A nanoclay incorporation on the morphology and gas permeation properties of P ebax2533 polymer. J. Appl. Polym. Sci. 2017, 134, 45302. [Google Scholar]
- Raine, T.P.; Istrate, O.M.; King, B.E.; Craster, B.; Kinloch, I.A.; Budd, P.M. Graphene/Polyamide Laminates for Supercritical CO2 and H2S Barrier Applications: An Approach toward Permeation Shutdown. Adv. Mater. Interfaces 2018, 5, 1800304. [Google Scholar] [CrossRef]
- Ait Cherif, G.; Kerkour, A.; Baouz, T.; Pillin, I.; Grohens, Y. Investigating the diffusional behaviour of Irganox® 1076 antioxidant in HDPE/Cloisite® 15A nanocomposite-based food contact packaging films: Effect of nanoclay loading. Packag. Technol. Sci. 2018, 31, 621–629. [Google Scholar] [CrossRef]
- Soltani, I.; Smith, S.D.; Spontak, R.J. Effect of polyelectrolyte on the barrier efficacy of layer-by-layer nanoclay coatings. J. Membr. Sci. 2017, 526, 172–180. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Abedi, B.; Bodaghi, H.; Davarynejad, G.; Haratizadeh, H.; Conte, A. Investigation of developed clay-nanocomposite packaging film on quality of peach fruit (Prunus persica Cv. Alberta) during cold storage. J. Food Process. Preserv. 2018, 42, e13466. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, M.H.; Ko, J.A.; Kang, D.H.; Bae, H.; Park, H.J. Influence of Food with High Moisture Content on Oxygen Barrier Property of Polyvinyl Alcohol (PVA)/Vermiculite Nanocomposite Coated Multilayer Packaging Film. J. Food Sci. 2018, 83, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yussuf, A.; Al-Saleh, M.; Al-Samhan, M.; Al-Enezi, S.; Al-Banna, A.; Abraham, G. Investigation of polypropylene-montmorillonite clay nanocomposite films containing a pro-degradant additive. J. Polym. Environ. 2018, 26, 275–290. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Fradique, R.; Correia, T.R.; Miguel, S.P.; de Sá, K.D.; Figueira, D.R.; Mendonça, A.G.; Correia, I.J. Production of new 3D scaffolds for bone tissue regeneration by rapid prototyping. J. Mater. Sci. Mater. Med. 2016, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Gutiérrez, M.; Ferrer, M.; del Monte, F. Preparation of Chitosan Nanocompositeswith a Macroporous Structure by Unidirectional Freezing and Subsequent Freeze-Drying. Mar. Drugs 2014, 12, 5619–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.C.; Gutiérrez, M.C.; del Monte, F. Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Håkansson, K.; Salajkova, M.; Lundell, F.; Wågberg, L.; Berglund, L.A. Highly Conducting, Strong Nanocomposites Based on Nanocellulose-Assisted Aqueous Dispersions of Single-Wall Carbon Nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Surudžić, R.; Janković, A.; Bibić, N.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Mišković-Stanković, V.; Park, S.J.; Rhee, K.Y. Physico–chemical and mechanical properties and antibacterial activity of silver/poly(vinyl alcohol)/graphene nanocomposites obtained by electrochemical method. Compos. Part B Eng. 2016, 85, 102–112. [Google Scholar] [CrossRef]
- Ivanova, A.; Fravventura, M.C.; Fattakhova-Rohlfing, D.; Rathouský, J.; Movsesyan, L.; Ganter, P.; Savenije, T.J.; Bein, T. Nanocellulose-Templated Porous Titania Scaffolds Incorporating Presynthesized Titania Nanocrystals. Chem. Mater. 2015, 27, 6205–6212. [Google Scholar] [CrossRef]
- Tsuge, Y.; Moriya, T.; Shiratori, S. Porous Transition of Polyelectrolyte Film through Reaction-Induced Phase Separation Caused by Interaction with Specific Metal Ions. Langmuir 2016, 32, 7219–7227. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Guo, Q.; Zhao, Y.; Zhang, P.; Zhang, T.; Zhang, X.; Li, C. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 25798–25807. [Google Scholar] [CrossRef] [PubMed]
- Pierchala, M.K.; Makaremi, M.; Tan, H.L.; Pushpamalar, J.; Muniyandy, S.; Solouk, A.; Lee, S.M.; Pasbakhsh, P. Nanotubes in nanofibers: Antibacterial multilayered polylactic acid/halloysite/gentamicin membranes for bone regeneration application. Appl. Clay Sci. 2018, 160, 95–105. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Chai, W.; Compaan, A.; Huang, Y. Self-Supporting Nanoclay as Internal Scaffold Material for Direct Printing of Soft Hydrogel Composite Structures in Air. ACS Appl. Mater. Interfaces 2017, 9, 17456–17465. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, A.; Afewerki, S.; Oklu, R.; Gaharwar, A.K.; Khademhosseini, A. Effect of ionic strength on shear-thinning nanoclay–polymer composite hydrogels. Biomater. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Jiang, H.; Haraguchi, K. Ultrastiff, Thermoresponsive Nanocomposite Hydrogels Composed of Ternary Polymer–Clay–Silica Networks. Macromolecules 2018, 51, 529–539. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. [Google Scholar] [CrossRef]
- Luo, Y.; Dolder, C.K.; Walker, J.M.; Mishra, R.; Dean, D.; Becker, M.L. Synthesis and Biological Evaluation of Well-Defined Poly(propylene fumarate) Oligomers and Their Use in 3D Printed Scaffolds. Biomacromolecules 2016, 17, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-S.; Cong, Z.-X.; Cao, J.-B.; Ke, K.-M.; Peng, Q.-L.; Gao, J.; Yang, H.-H.; Liu, G.; Chen, X. Multifunctional Fe3O4@Polydopamine Core–Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-Initiated Polymer Brushes in the Biomedical Field: Applications in Membrane Science, Biosensing, Cell Culture, Regenerative Medicine and Antibacterial Coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Knopfmacher, O.; Hammock, M.L.; Appleton, A.L.; Schwartz, G.; Mei, J.; Lei, T.; Pei, J.; Bao, Z. Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 2014, 5, 2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, Y.; Jiang, W.; Yin, J.; Tang, Q.; Yang, X. Parallel Carbon Nanotube Stripes in Polymer Thin Film with Remarkable Conductive Anisotropy. ACS Appl. Mater. Interfaces 2014, 6, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-C.; Wang, X.; Gong, L.-X.; Peng, K.; Zhao, L.; Chen, Q.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Creep and recovery of polystyrene composites filled with graphene additives. Compos. Sci. Technol. 2014, 91, 63–70. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Gu, S.-X.; Li, W.-W.; Chen, T.-T.; Xu, Q.; Wang, W.J.B. A paper disk equipped with graphene/polyaniline/Au nanoparticles/glucose oxidase biocomposite modified screen-printed electrode: Toward whole blood glucose determination. Biosens. Bioelectron. 2014, 56, 77–82. [Google Scholar] [CrossRef]
- Turkmen, E.; Bas, S.Z.; Gulce, H.; Yildiz, S.J.E.A. Glucose biosensor based on immobilization of glucose oxidase in electropolymerized poly(o-phenylenediamine) film on platinum nanoparticles-polyvinylferrocenium modified electrode. Electrochim. Acta 2014, 123, 93–102. [Google Scholar] [CrossRef]
- Mishra, D.K.; Yadav, K.S.; Prabhakar, B.; Gaud, R.S. Nanocomposite for cancer targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A.M.A., Mohammad, A., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 323–337. 5p. [Google Scholar]
- Rajan, M.; Murugan, M.; Ponnamma, D.; Sadasivuni, K.K.; Munusamy, M.A. Poly-carboxylic acids functionalized chitosan nanocarriers for controlled and targeted anti-cancer drug delivery. Biomed. Pharmacother. 2016, 83, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Attanasio, G.; Izzo, L.; Sorrentino, A. Controlled release mechanisms of sodium benzoate from a biodegradable polymer and halloysite nanotube composite. Polym. Int. 2017, 66, 690–698. [Google Scholar] [CrossRef]
- Saha, N.R.; Sarkar, G.; Roy, I.; Rana, D.; Bhattacharyya, A.; Adhikari, A.; Mukhopadhyay, A.; Chattopadhyay, D. Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr. Polym. 2016, 136, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Vladisavljević, G.T.; Thomas, N.L.; Nagy, Z.K. Fabrication of composite poly(d,l-lactide)/montmorillonite nanoparticles for controlled delivery of acetaminophen by solvent-displacement method using glass capillary microfluidics. Colloids Surf. B Biointerfaces 2016, 141, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharif, M.; Mohamed, W.S. Preparation and Characterization of Melt Spun Polypropylene/Montmorillonite Nanocomposite Fibre for Ibuprofen Drug Delivery application. Egypt. J. Chem. 2018, 61, 235–244. [Google Scholar]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Di Muzio, L.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S. Design of a tunable nanocomposite double network hydrogel based on gellan gum for drug delivery applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unuabonah, E.I.; Taubert, A. Clay–polymer nanocomposites (CPNs): Adsorbents of the future for water treatment. Appl. Clay Sci. 2014, 99, 83–92. [Google Scholar] [CrossRef]
- Hernández-Hernández, K.A.; Illescas, J.; Díaz-Nava, M.C.; Muro-Urista, C.R.; Martínez-Gallegos, S.; Ortega-Aguilar, R.E. Polymer-clay nanocomposites and composites: Structures, characteristics, and their applications in the removal of organic compounds of environmental interest. Med. Chem. 2016, 6, 201–210. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Issa, Z.A.; Oumi, A.B. Synthesis and application of magnetite polyacrylamide amino-amidoxime nano-composites as adsorbents for water pollutants. J. Polym. Res. 2016, 23, 69. [Google Scholar] [CrossRef]
- Kara, A.; Tekin, N.; Alan, A.; Şafaklı, A. Physicochemical parameters of Hg(II) ions adsorption from aqueous solution by sepiolite/poly(vinylimidazole). J. Environ. Chem. Eng. 2016, 4, 1642–1652. [Google Scholar] [CrossRef]
- Yildiz, G.; Senkal, B.F. Formation of composites between polyvinylimidazole and bentonites and their use for removal of remazol black B from water. Sep. Sci. Technol. 2016, 51, 2596–2603. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Xiao, C.; Shao, D.; Xu, Z.; Wang, J.; Hu, S.; Li, X.; Wang, W. Polyaniline (PANI) modified bentonite by plasma technique for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2017, 411, 331–337. [Google Scholar] [CrossRef]
- El-Korashy, S.A.; Elwakeel, K.Z.; El-Hafeiz, A.A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb(II), Mn(VII) and Cr(VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; El Akili, C. Performances of local chitosan and its nanocomposite 5% Bentonite/Chitosan in the removal of chromium ions (Cr (VI)) from wastewater. Int. J. Boil. Macromol. 2018, 108, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.-A. Nanoplastics in the Aquatic Environment. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–399. 8p. [Google Scholar]
- Noventa, S.; Hacker, C.; Rowe, D.; Elgy, C.; Galloway, T. Dissolution and bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: An in vivo study with oyster embryos. Nanotoxicology 2018, 12, 63–78. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total. Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, Y.; Deng, F.; Cho, U.R. Effects of silane coupling agents on tribological properties of bentonite/nitrile butadiene rubber composites. Appl. Clay Sci. 2017, 38, 2347–2357. [Google Scholar] [CrossRef]
- Miller, L.; Soulliere, K.; Sawyer-Beaulieu, S.; Tseng, S.; Tam, E. Challenges and Alternatives to Plastics Recycling in the Automotive Sector. Materials 2014, 7, 5883–5902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Yang, S.; Zhao, X.; Du, P.; Xiong, J. Electrospun montmorillonite modified poly(vinylidene fluoride) nanocomposite separators for lithium-ion batteries. Mater. Res. Bull. 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Liu, E.; Sarkar, B.; Wang, L.; Naidu, R. Copper-complexed clay/poly-acrylic acid composites: Extremely efficient adsorbents of ammonia gas. Appl. Clay Sci. 2016, 121–122, 154–161. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.; Evtugyn, V.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.; Fakhrullin, R. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Holder, K.M.; Ruiz, S.; Hahn, W.; Song, Y.; Lvov, Y.M.; Grunlan, J.C. Environmentally Benign Halloysite Nanotube Multilayer Assembly Significantly Reduces Polyurethane Flammability. Adv. Funct. Mater. 2018, 28, 1703289. [Google Scholar] [CrossRef]






| Clay Layer Type | Clay Group | Clay Species |
|---|---|---|
| 1:1 | Rectorite, Kaolinite, Halloysite, Chyrsotile | Lizardite, Berthierine, Cronstedtite, Kellyite, Fraipontite, Brindleyite, Dickite, Nacrite |
| 2:1 | Smectite, Vermiculite, Pyrophylite talc, Mica, Brittle Mica | Montmorillonite, Laponite, Sepiolite, Hectorite, Bentonite, Vermiculite, Pyrophyllite, Talc, Muscovite, Paragonite, Clintonite, Bityite |
| 2:1:1 | Chlorite | Amesite, Cookeite |
| Nanoclay | Polymer | Reference |
|---|---|---|
| Co-Al-LDH | PS | [67] |
| Cloisite® 30B | Chitosan/polylactide | [68] |
| Mg-Al-LDH | Epoxy | [69] |
| Mg-Al-LDH | Acrylic resin | [70] |
| Cloisite and sepiolite | Hydrogenated nitrile butadiene rubber | [71] |
| Nanoclay | Polymer | Clay Structure | Reference |
|---|---|---|---|
| Cloisite® 30B | Nylon 6 | Intercalated | [93] |
| Na-MMT | Nylon 66 | Disordered | [94] |
| Na-MMT | Epoxy | Intercalated | [95] |
| Na-MMT | Polyamide | Exfoliated | [96] |
| MMT | Polypropylene | Exfoliated | [97] |
| MMT | Polyvinyl chloride | Exfoliated | [98] |
| Nanoclay | Polymer | Clay Structure | Reference |
|---|---|---|---|
| MMT | 2,2,2-trifluoroethyl methacrylate | Exfoliated and intercalated | [35] |
| Fe-kanemite | PPy | Exfoliated | [111] |
| MMT | Bisphenol A polycarbonate | Exfoliated | [112] |
| Vinyl clay | PS | Intercalated | [113] |
| Sepiolite | PP | Exfoliated and intercalated | [101] |
| MMT | PP | Exfoliated | [114] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091696
Guo F, Aryana S, Han Y, Jiao Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Applied Sciences. 2018; 8(9):1696. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091696
Chicago/Turabian StyleGuo, Feng, Saman Aryana, Yinghui Han, and Yunpeng Jiao. 2018. "A Review of the Synthesis and Applications of Polymer–Nanoclay Composites" Applied Sciences 8, no. 9: 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/app8091696