Edible Oils as Practical Phase Change Materials for Thermal Energy Storage
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
3. Results and Discussion
3.1. Thermal Properties of Margarine and Shortening
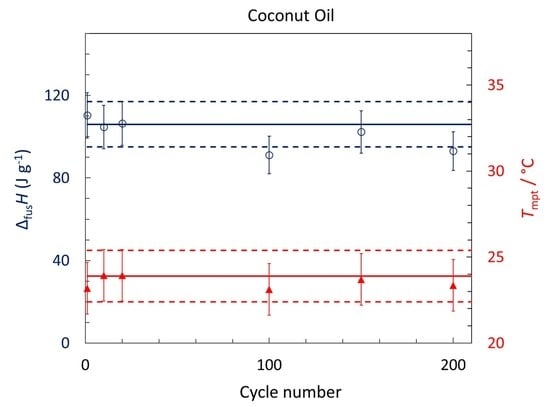
3.2. Thermal Properties of Coconut Oil
3.3. Thermal Energy Storage with Coconut Oil in a Residential Greenhouse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noël, J.A.; Kahwaji, S.; Degrosseilliers, L.; Groulx, D.; White, M.A. Phase Change Materials. In Storing Energy: With Special Reference to Renewable Energy Sources; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 249–272. ISBN 9780128034408. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L. Heat and Cold Storage with PCM; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540685562. [Google Scholar]
- Suppes, G.J.J.; Goff, M.J.J.; Lopes, S. Latent heat characteristics of fatty acid derivatives pursuant phase change material applications. Chem. Eng. Sci. 2003, 58, 1751–1763. [Google Scholar] [CrossRef]
- Rozanna, D.; Chuah, T.G.; Salmiah, A.; Choong, T.S.Y.; Sa’ari, M. Fatty Acids as Phase Change Materials (PCMs) for Thermal Energy Storage: A Review. Int. J. Green Energy 2005, 1, 495–513. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Nikolić, R.; Marinović-Cincović, M.; Gadžurić, S.; Zsigrai, I. New materials for solar thermal storage—Solid/liquid transitions in fatty acid esters. Sol. Energy Mater. Sol. Cells 2003, 79, 285–292. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D. Low chain esters of stearic acid as phase change materials for thermal energy storage in buildings. Sol. Energy Mater. Sol. Cells 1995, 36, 311–322. [Google Scholar] [CrossRef]
- Alper Aydin, A. High-chain fatty acid esters of 1-octadecanol as novel organic phase change materials and mathematical correlations for estimating the thermal properties of higher fatty acid esters’ homologous series. Sol. Energy Mater. Sol. Cells 2013, 113, 44–51. [Google Scholar] [CrossRef]
- Peippo, K.; Kauranen, P.; Lund, P.D. A multicomponent PCM wall optimized for passive solar heating. Energy Build. 1991, 17, 259–270. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D.W. Development and application of organic phase change mixtures in thermal storage gypsum wallboard. Sol. Energy Mater. Sol. Cells 1995, 36, 147–157. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Stable, low-cost phase change material for building applications: The eutectic mixture of decanoic acid and tetradecanoic acid. Appl. Energy 2016, 168, 457–464. [Google Scholar] [CrossRef]
- Gervajio, G.C. Fatty Acids and Derivatives from Coconut Oil. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9780471678496. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Otamiri, F.O.; Ogugua, V.N.; Joshua, P.E.; Odiba, A.S.; Ukegbu, C.Y. Physicochemical Characterization of Coconut Copra (Dry Flesh) oil and Production of Biodiesel from Coconut Copra Oil. Jökull J. Univ. Niger. Nsukka 2014, 64, 201–236. [Google Scholar]
- Noël, J.A.; Allred, P.M.; White, M.A. Life cycle assessment of two biologically produced phase change materials and their related products. Int. J. Life Cycle Assess. 2014, 20, 367–376. [Google Scholar] [CrossRef]
- PureTemp LLC-Global Authority on Phase Change Material. Available online: http://www.puretemp.com/ (accessed on 27 January 2017).
- Phase Change Material Products Limited. Available online: http://www.pcmproducts.net/Phase_Change_Material_Products.htm (accessed on 18 February 2019).
- Ceres Greenhouse Solutions. Available online: https://ceresgs.com/climate-control/phase-change/ (accessed on 18 February 2019).
- Kahwaji, S.; White, M.A. Prediction of the properties of eutectic fatty acid phase change materials. Thermochim. Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Data supporting the prediction of the properties of eutectic organic phase change materials. Data Br. 2018, 17, 724–730. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture—National Nutrient Database for Standard Reference 1 Release April. 2018; Basic Report: 04047, Oil, Coconut. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 18 February 2019).
- Prapun, R.; Cheetangdee, N.; Udomrati, S. Characterization of virgin coconut oil (VCO) recovered by different techniques and fruit maturities. Int. Food Res. J. 2016, 23, 2117–2124. [Google Scholar]
- Srivastava, Y.; Semwal, A.D.; Sajeevkumar, V.A.; Sharma, G.K. Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J. Food Sci. Technol. 2017, 54, 45–54. [Google Scholar] [CrossRef]
- Tan, C.; Che Man, Y. Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: Effects of scanning rate variation. Food Chem. 2002, 76, 89–102. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P. Coconut oil as base oil for industrial lubricants—Evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Moigradean, D.; Poiana, M.-A.; Gogoasa, I. Quality characteristics and oxidative stability of coconut oil during storage. J. Agroaliment. Process. Technol. 2012, 18, 272–276. [Google Scholar]
- Putri, W.A.; Fahmi, Z.; Sutjahja, I.M.; Kurnia, D.; Wonorahardjo, S. Thermophysical parameters of coconut oil and its potential application as the thermal energy storage system in Indonesia. J. Phys. Conf. Ser. 2016, 739. [Google Scholar] [CrossRef]
- Saleel, C.A.; Mujeebu, M.A.; Algarni, S. Coconut oil as phase change material to maintain thermal comfort in passenger vehicles. J. Therm. Anal. Calorim. 2018. [Google Scholar] [CrossRef]
- Mettawee, E.S.; Ead, A.I. Energy Saving in Building with Latent Heat Storage. Int. J. Therm. Environ. Eng. 2013, 5, 21–30. [Google Scholar]
- Wonorahardjo, S.; Sutjahja, I.; Kurnia, D.; Fahmi, Z.; Putri, W. Potential of Thermal Energy Storage Using Coconut Oil for Air Temperature Control. Buildings 2018, 8, 95. [Google Scholar] [CrossRef]
- Tipvarakarnkoon, T.; Blochwitz, R.; Senge, B. Rheological properties and phase change behaviors of coconut fats and oils. Annu. Trans. Nord. Rheol. Soc. 2008, 16, 159–166. [Google Scholar]
- Al-Jethelah, M.; Ebadi, S.; Venkateshwar, K.; Tasnim, S.H.H.; Mahmud, S.; Dutta, A. Charging nanoparticle enhanced bio-based PCM in open cell metallic foams: An experimental investigation. Appl. Therm. Eng. 2019, 148, 1029–1042. [Google Scholar] [CrossRef]
- Németh, B.; Németh, Á.S.; Ujhidy, A.; Tóth, J.; Trif, L.; Gyenis, J.; Feczkó, T. Fully bio-originated latent heat storing calcium alginate microcapsules with high coconut oil loading. Sol. Energy 2018, 170, 314–322. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, S.-G.; Chang, S.; Kang, Y.; Wi, S.; Kim, S. Thermal Performance Evaluation of Fatty Acid Ester and Paraffin Based Mixed SSPCMs Using Exfoliated Graphite Nanoplatelets (xGnP). Appl. Sci. 2016, 6, 106. [Google Scholar] [CrossRef]
- Wi, S.; Seo, J.; Jeong, S.-G.; Chang, S.J.; Kang, Y.; Kim, S. Thermal properties of shape-stabilized phase change materials using fatty acid ester and exfoliated graphite nanoplatelets for saving energy in buildings. Sol. Energy Mater. Sol. Cells 2015, 143, 168–173. [Google Scholar] [CrossRef]
- Agarwal, R.K. Extraction Processes of Virgin Coconut Oil. MOJ Food Process. Technol. 2017, 4, 54–56. [Google Scholar] [CrossRef]
- Villarino, B.J.; Dy, L.M.; Lizada, M.C.C. Descriptive sensory evaluation of virgin coconut oil and refined, bleached and deodorized coconut oil. LWT Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Cassel, R.B. Tzero Technology and Linearity; TA Instruments-TA325; TA Instruments: New Castle, DE, USA, 2005. [Google Scholar]
- Johnson, M.B.; White, M.A. Thermal Methods. In Multi Length-Scale Characterization; Bruce, D.W., O’Hare, D., Walton, R.I., Eds.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Höhne, G.; Hemminger, W.; Flammersheim, H.J. Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 9783540004677. [Google Scholar]
- ASTM Standard E1269-11. Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty Acids and Related Phase Change Materials for Reliable Thermal Energy Storage at Moderate Temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Feldman, D.; Shapiro, M.M.; Banu, D.; Fuks, C.J. Fatty acids and their mixtures as phase-change materials for thermal energy storage. Sol. Energy Mater. 1989, 18, 201–216. [Google Scholar] [CrossRef]
- Hawes, D.W.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Passive thermal control in residential buildings using phase change materials. Renew. Sustain. Energy Rev. 2016, 55, 371–398. [Google Scholar] [CrossRef]
- Greenhouse Megastore. Available online: https://www.greenhousemegastore.com/structures/greenhouse-kits/ (accessed on 18 February 2019).
- Greenhouses Canada. Available online: http://www.greenhousescanada.com/Palram-Glory-Grey-8-x-12-10mm-TwinWall-Glazing.html (accessed on 18 February 2019).
- Boulard, T.; Razafinjohany, E.; Baille, A.; Jaffrin, A.; Fabre, B. Performance of a greenhouse heating system with a phase change material. Agric. For. Meteorol. 1990, 52, 303–318. [Google Scholar] [CrossRef]
- Dutil, Y.; Rousse, D.; Lassue, S.; Zalewski, L.; Joulin, A.; Virgone, J.; Kuznik, F.; Johannes, K.; Dumas, J.-P.; Bédécarrats, J.-P.; et al. Modeling phase change materials behavior in building applications: Comments on material characterization and model validation. Renew. Energy 2014, 61, 132–135. [Google Scholar] [CrossRef]
- Dutil, Y.; Rousse, D.R.; Salah, B.; Phane Lassue, S.; Zalewski, L. A review on phase-change materials: Mathematical modeling and simulations. Renew. Sustain. Energy Rev. 2010, 15, 112–130. [Google Scholar] [CrossRef]
- Charvát, P.; Klimeš, L.; Ost, M. Numerical and experimental investigation of a PCM-based thermal storage unit for solar air systems. Energy Build. 2014, 68, 488–497. [Google Scholar] [CrossRef]
- Mavrigiannaki, A.; Ampatzi, E. Latent heat storage in building elements: A systematic review on properties and contextual performance factors. Renew. Sustain. Energy Rev. 2016, 60, 852–866. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, A.; Behzadi, S.; Chen, J.J.J.; Farid, M.M. Computer simulation and experimental measurments for an experimental PCM-impregnated office building. In Proceedings of the Building Simulation 2011: 12th Conference of International Building Performance Simulation Association, Sydney, Australia, 14–16 November 2011; pp. 56–63. [Google Scholar]
- Chase, M.W. NIST-JANAF Themochemical Tables, Fourth Edition. (Journal of Physical and Chemical Reference Data Monographs); NIST: Gaithersburg, MD, USA, 1998; pp. 1–1951. [Google Scholar]
- Noureddini, H.; Teoh, B.C.; Clements, L.D.C. Densities of vegetable oils and Fatty Acids. Chem. Biomol. Eng. Res. Publ. 1992, 69, 1184–1188. [Google Scholar] [CrossRef]
- Bulk Apothecary—Refined Coconut Oil. Available online: https://www.bulkapothecary.com/raw-ingredients/bulk-natural-oils/coconut-oil-76-degree/ (accessed on 18 February 2019).
- IndexMundi. Available online: https://www.indexmundi.com/commodities/?commodity=coconut-oil (accessed on 19 February 2019).
- Fabiani, C.; Pisello, A.; Barbanera, M.; Cabeza, L.; Cotana, F.; Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F.; Cotana, F. Assessing the Potentiality of Animal Fat Based-Bio Phase Change Materials (PCM) for Building Applications: An Innovative Multipurpose Thermal Investigation. Energies 2019, 12, 1111. [Google Scholar] [CrossRef]







| Product | Abbreviation | Cost (US $/kg) | Main Composition |
|---|---|---|---|
| Parkay® Margarine | M1 | 2 | Non-hydrogenated oil blend (68%), modified milk ingredients (18%) and water (12%) |
| Imperial® Margarine | M2 | 2 | Non-hydrogenated oil blend (60%), milk ingredients and water (unspecified amounts) |
| Crisco® Vegetable Shortening | VS | 4 | Fully and partially hydrogenated soybean and palm oils, mono and diglycerides |
| Refined Coconut Oil (Suraj® brand) | R-CNO | 6 | Coconut oil |
| Virgin Coconut Oil (Our Finest® brand) | V-CNO | 11 | 100% virgin coconut oil |
| Reference | Measurement Method | ΔfusH/(J g−1) | Tmpt /°C |
|---|---|---|---|
| This work–refined coconut oil (R-CNO) | DSC | 105 ± 11 | 24.5 ± 1.5 |
| This work–virgin coconut oil (V-CNO) | DSC | 106 ± 11 | 23.9 ± 1.5 |
| [25] | DSC | 72.01 ± 0.21 | 24.73 ± 0.12 |
| [26] | DSC | 120.6 ± 2.0 | 21.05 |
| [29] | T-history | 249 | not reported |
| [33] | DSC | 97 - 102 | 26.2–26.7 |
| [34] | DSC | 103 ± 1 | 24 ± 1 |
| [35] | DSC | 115.3 | 23.9 |
| [36] | DSC | 110.4 | 26.78 |
| Sample | ΔfusH /J g−1 | Tmpta /°C | Comment |
|---|---|---|---|
| M1 | 8 ± 1 | 35.5 ± 1.5 | Small ΔfusH value. Thermally unstable after the first melt. Supercools by about 20 K in the DSC. |
| M2 | 7 ± 1 | 33.0 ± 1.5 °C | Small ΔfusH value. Thermally unstable after the first melt. Supercools by about 20 K in the DSC. |
| VS | 12 ± 1 | 44.8 ± 1.5 | Small ΔfusH value. Better thermal stability than margarine. Supercools by about 15 K in the DSC. |
| R-CNO | 105 ± 11 | 24.5 ± 1.5 | Large ΔfusH. Thermally stable after 200 melt-freeze cycle. Supercooling decreases as the cooling rate decreases, and can be negligible for a bulk sample. Cp,s = 1.6 ± 0.2 J K−1 mol−1; Cp,l = 2.2 ± 0.2 J K−1 mol−1; κs = 0.19 ± 0.2 W m−1 K−1; κl = 0.17 ± 0.2 W m−1 K−1. |
| V-CNO | 106 ± 11 | 23.9 ± 1.5 | Large ΔfusH. Thermally stable after 200 melt-freeze cycle. Supercooling decreases as the cooling rate decreases. Thermal properties similar to those of R-NCO. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahwaji, S.; White, M.A. Edible Oils as Practical Phase Change Materials for Thermal Energy Storage. Appl. Sci. 2019, 9, 1627. https://0-doi-org.brum.beds.ac.uk/10.3390/app9081627
Kahwaji S, White MA. Edible Oils as Practical Phase Change Materials for Thermal Energy Storage. Applied Sciences. 2019; 9(8):1627. https://0-doi-org.brum.beds.ac.uk/10.3390/app9081627
Chicago/Turabian StyleKahwaji, Samer, and Mary Anne White. 2019. "Edible Oils as Practical Phase Change Materials for Thermal Energy Storage" Applied Sciences 9, no. 8: 1627. https://0-doi-org.brum.beds.ac.uk/10.3390/app9081627