Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils
Abstract
:1. Introduction
2. Materials and Methods
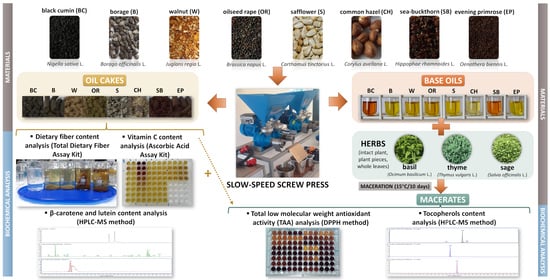
2.1. Materials and Experiment Design
2.2. Biochemical Analysis
2.2.1. Tocopherols, β-Carotene and Lutein
2.2.2. Total Low Molecular Weight Antioxidant Activity (TAA)
2.2.3. Total Dietary Fibre
2.2.4. Vitamin C
2.3. Statistical Analysis
3. Results
3.1. Total Tocopherols in Oils After the Addition of Herbs
3.2. Total Low Molecular Weight Antioxidant Activity (TAA) of Oils After Addition of Herbs
3.3. Selected Chemical Components in Oil Cakes of Eight Plant Species After Cold-Press of Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Standard for Edible Fats and Oils Not Covered by Individual Standards. In Codex Alimentarius: Fats, Oils and Related Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; pp. 2–7. [Google Scholar]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Parry, J.W.; Cheng, Z.; Moore, J.; Yu, L.L. Fatty acid composition, antioxidant properties, and antiproliferative capacity of selected cold-pressed seed flours. J. Am. Oil Chem. Soc. 2008, 85, 457–464. [Google Scholar] [CrossRef]
- Siger, A.; Józefiak, M.; Górnaś, P. Cold-pressed and hot-pressed rapeseed oil: The effects of roasting and seed moisture on the antioxi- dant activity, canolol, and tocopherol level. Acta Sci. Pol. Technol. Aliment. 2017, 16, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kania, M.; Michalak, M.; Gogolewski, M. Antioxidative Potential of Substances. Acta Sci. Pol. Technol. Aliment. 2004, 3, 113–121. [Google Scholar]
- Tasan, M.; Demirci, M. Total and individual tocopherol contents of sunflower oil at different steps of refining. Eur. Food Res. Technol. 2005, 220, 251–254. [Google Scholar] [CrossRef]
- Verhé, R.; Verleyen, T.; Van Hoed, V.; De Greyt, W. Influence of Refining of Vegetable Oils. J. Oil Palm Res. 2006, 4, 168–179. [Google Scholar]
- Medina-Juárez, L.Á.; Gamez-Mez, N. Effect of Refining Process and Use of Natural Antioxidants on Soybean Oil. In Soybean: Biochemistry, Chemistry and Physiology; Ng, T.-B., Ed.; InTech: London, UK, 2011; pp. 435–460. ISBN 9789533072197. [Google Scholar]
- Wu, Y.; Zhou, R.; Wang, Z.; Wang, B.; Yang, Y.; Ju, X.; He, R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, F.; Li, Y.; Luo, X.; Wang, X.; Wang, C.; Wen, B.; Guan, X.; Xu, Y.; Liu, B. Effect of the chemical refining process on composition and oxidative stability of evening primrose oil. J. Food Process. Preserv. 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Mert, H.; İrak, K.; Çibuk, S.; Yıldırım, S.; Mert, N. The effect of evening primrose oil (Oenothera biennis) on the level of adiponectin and some biochemical parameters in rats with fructose induced metabolic syndrome. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Mayor, A.B.R. Prooxidant effect of the crude ethanolic leaf extract of Ficus odorata Blanco Merr. in vitro: Its medical significance. Int. J. Biotechnol. Bioeng. 2014, 8, 53–60. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Pal, D. Natural compounds extracted from medicinal plants and their immunomodulatory activities. In Bioactive Natural Products for Pharmaceutical Applications. Advanced Structured Materials; Pal, D., Nayak, A.K., Eds.; Springer: Cham, Switzerland, 2021; Volume 140, pp. 197–261. [Google Scholar]
- Saravanakumar, A.; Periyasamy, P.; Jang, H.T. In vitro assessment of three different artemisia species for their antioxidant and anti-fibrotic activity. Biocatal. Agric. Biotechnol. 2019, 18, 101040. [Google Scholar] [CrossRef]
- Benot-Dominguez, R.; Tupone, M.G.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Quintiliani, M.; Cinque, B.; Forte, I.M.; Cifone, M.G.; Ippoliti, R.; et al. Olive leaf extract impairs mitochondria by pro-oxidant activity in MDA-MB-231 and OVCAR-3 cancer cells. Biomed. Pharmacother. 2021, 134, 111139. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H. Identification of Plant Extracts that Inhibit Cellular Senescence in Human Fibroblasts, Endothelial Cells, and Vascular Smooth Muscle Cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 584–592. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. NPJ Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Nogala-Kalucka, M.; Rudzinska, M.; Zadernowski, R.; Siger, A.; Krzyzostaniak, I. Phytochemical content and antioxidant properties of seeds of unconventional oil plants. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1481–1487. [Google Scholar] [CrossRef]
- Aydeniz, B.; Güneşer, O.; Yılmaz, E. Physico-chemical, sensory and aromatic properties of cold press produced safflower oil. J. Am. Oil Chem. Soc. 2014, 91, 99–110. [Google Scholar] [CrossRef]
- Fernández-Cuesta, Á.; Velasco, L.; Ruiz-Méndez, M.V. Novel safflower oil with high γ-tocopherol content has a high oxidative stability. Eur. J. Lipid Sci. Technol. 2014, 116, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Sielicka, M.; Małecka, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-tocopherol) levels in plant oils. Flavour Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Alenzi, F.Q.; El-Bolkiny, Y.E.-S.; Salem, M.L. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br. J. Biomed. Sci. 2010, 67, 20–28. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Agbaria, R.; Gabarin, A.; Dahan, A.; Ben-Shabat, S. Anticancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinone composition of the seed. Drug Des. Dev. Ther. 2015, 9, 3119–3124. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.; Khan, R.S.; Hussain, M.I.; Farooq, M.; Ahmad, A.; Ahmed, I. A comprehensive characterisation of safflower oil for its potential applications as a bioactive food ingredient—A review. Trends Food Sci. Technol. 2017, 66, 176–186. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; El-qousy, S.M.; El-Ghlban, S.; Moawad, H.F. Role of borage seed oil and fish oil with or without turmeric and alpha-tocopherol in prevention of cardiovascular disease and fatty liver in rats. J. Oleo Sci. 2018, 67, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Bawari, S.; Patni, P.; Sah, A.N. Chapter 3.7—Borage (Borago officinalis L.). In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 165–170. ISBN 9780128124918. [Google Scholar]
- De Spirt, S.; Stahl, W.; Tronnier, H.; Sies, H.; Bejot, M.; Maurette, J.-M.; Heinrich, U. Intervention with flaxseed and borage oil supplements modulates skin condition in women. Br. J. Nutr. 2008, 101, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid profile of new promising unconventional plant oils for cosmetic use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrzab, A.; Jarocka-Karpowicz, I.; Muszyńska, M.; Skrzydlewska, E. The effect of sea buckthorn (Hippophae rhamnoides L.) seed oil on UV-induced changes in lipid metabolism of human skin cells. Antioxidants 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty Acid Composition and Oxidative Stability of Cold-pressed Edible Seed Oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Youdim, K.A.; Damien Dorman, H.J.; Deans, S.G. The antioxidant effectiveness of thyme oil, α-tocopherol and ascorbyl palmitate on evening primrose oil oxidation. J. Essent. Oil Res. 1999, 11, 643–648. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Ghadermazi, R.; Keramat, J.; Goli, S.A.H. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L.) and sage (Salvia officinalis L.) essential oils in various model systems. Int. Food Res. J. 2017, 24, 1628–1635. [Google Scholar]
- Sielicka, M.; Małecka, M. Enhancement of oxidative stability of flaxseed oil through flaxseed, evening primrose and black cumin cake extracts. J. Food Process. Preserv. 2017, 41, e13070. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [Green Version]
- Soleimanifar, M.; Niazmand, R.; Jafari, S.M. Evaluation of oxidative stability, fatty acid profile, and antioxidant properties of black cumin seed oil and extract. J. Food Meas. Charact. 2019, 13, 383–389. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarshenas, M.M.; Sohrabpour, M.; Zargaran, A. Herbal medicinal oils in traditional Persian medicine. Pharm. Biol. 2013, 51, 1208–1218. [Google Scholar] [CrossRef]
- Kantawong, F.; Singhatong, S.; Srilamay, A.; Boonyuen, K.; Mooti, N.; Wanachantararak, P.; Kuboki, T. Properties of macerated herbal oil. BioImpacts 2017, 7, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peschel, W.; Dieckmann, W.; Sonnenschein, M.; Plescher, A. High antioxidant potential of pressing residues from evening primrose in comparison to other oilseed cakes and plant antioxidants. Ind. Crop. Prod. 2007, 25, 44–54. [Google Scholar] [CrossRef]
- Sunil, L.; Appaiah, P.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Preparation of food supplements from oilseed cakes. J. Food Sci. Technol. 2015, 52, 2998–3005. [Google Scholar] [CrossRef] [Green Version]
- Serrapica, F.; Masucci, F.; Raffrenato, E.; Sannino, M.; Vastolo, A.; Barone, C.M.A.; Di Francia, A. High fiber cakes from mediterranean multipurpose oilseeds as protein sources for ruminants. Animals 2019, 9, 918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, S.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Krzewska, M.; Zieliński, K.; Fodor, J.; Janowiak, F. Glutathione provides antioxidative defence and promotes microspore-derived embryo development in isolated microspore cultures of triticale (× Triticosecale Wittm.). Plant Cell Rep. 2019, 38, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- López Ortíz, C.M.; Prats Moya, M.S.; Berenguer Navarro, V. A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J. Food Compos. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- Dolde, D.; Vlahakis, C.; Hazebroek, J. Tocopherols in breeding lines and effects of planting location, fatty acid composition, and temperature during development. J. Am. Oil Chem. Soc. 1999, 76, 349–355. [Google Scholar] [CrossRef]
- Syväoja, E.L.; Pilronen, V.; Varo, P.; Koivistoinen, P.; Salminen, K. Tocopherols and tocotrienols in Finnish foods: Oils and fats. J. Am. Oil Chem. Soc. 1986, 63, 328–329. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V.; Rius, I.A. Liquid chromatographic determination of tocopherols and tocotrienols in vegetable oils, formulated preparations, and biscuits. J. AOAC Int. 2000, 83, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.D.L.; Almeida, F.N.D.S.; Dimenstein, R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; de Camargo, A. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Fabrikov, D.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Rodríguez-García, I.; Gómez-Mercado, F.; Urrestarazu, M.; Lao, M.T.; Rincón-Cervera, M.Á.; Álvaro, J.E.; Lyashenko, S. Borage oil: Tocopherols, sterols and squalene in farmed and endemic-wild Borago species. J. Food Compos. Anal. 2019, 83, 103299. [Google Scholar] [CrossRef]
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Christie, W.W. The analysis of evening primrose oil. Ind. Crops Prod. 1999, 10, 73–83. [Google Scholar] [CrossRef]
- FAO/WHO. Summary of the role of vitamin E in human metabolic processes. In FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; pp. 121–131. [Google Scholar]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, M.F. Cold Pressed Oils, 1st ed.; Academic Press: London, UK, 2020; ISBN 978-0-12-818188-1. [Google Scholar]
- Hossain, M.B.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Application of response surface methodology to optimize pressurized liquid extraction of antioxidant compounds from sage (Salvia officinalis L.), basil (Ocimum basilicum L.) and thyme (Thymus vulgaris L.). Food Funct. 2010, 1, 269. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Orčić, D.; Lesjak, M.; Šibul, F. Essential oils as powerful antioxidants: Misconception or scientific fact? In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization, ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1218, pp. 187–208. [Google Scholar]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.; Kowalska, G.; Pankiewicz, U.; Włodarczyk-Stasiak, M.; Sujka, M.; Mazurek, A. Effect of rapeseed oil aromatisation with marjoram on the content of volatile fraction and antioxidant properties. J. Food Sci. Technol. 2020, 57, 1138–1149. [Google Scholar] [CrossRef] [Green Version]
- Şahin, S.; Elhussein, E.A.A. Valorization of a biomass: Phytochemicals in oilseed by-products. Phytochem. Rev. 2018, 17, 657–668. [Google Scholar] [CrossRef]
- Shahidi, F.; Amarowicz, R.; He, Y.; Wettasinghe, M. Antioxidant activity of phenolic extracts of evening primrose (Oenothera biennis): A preliminary study. J. Food Lipids 1997, 4, 75–86. [Google Scholar] [CrossRef]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crop. Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Silva-Flores, P.G.; Pérez-López, L.A.; Rivas-Galindo, V.M.; Paniagua-Vega, D.; Galindo-Rodríguez, S.A.; Álvarez-Román, R. Simultaneous GC-FID quantification of main components of rosmarinus officinalis l. and lavandula dentata essential oils in polymeric nanocapsules for antioxidant application. J. Anal. Methods Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]



| Type of Material | Characteristics |
|---|---|
| Raw Material | Seeds: black cumin (Nigella sativa L.), borage (Borago officinalis L.), evening primrose (Oenothera biennis L.), safflower (Carthamus tinctorius L), walnut (Juglans regia L)., common hazel (Corylus avellana L.), and oilseed rape (Brassica napus L.) Berries: sea-buckthorn (Hippophae rhamnoides L.) (first macerated with rapeseed oil in a 1:1 proportion) |
| Base Oils (Control Oils) | 8 base oils cold-pressed form seeds and berries at slow-speed screw press (temperature range of 33–35 °C) |
| Oil Cakes | 8 oil cakes-residue from the oil pressing process |
| Herbal Material (Herbs) Used for Preparation of Macerates | 3 herbs: sage (Salvia officinalis L.), common thyme (Thymus vulgaris L.), basil (Ocimum basilicum L.) Forms and weights of herbal material:
|
| Macerates (Base Oil + Herb) | 144 macerates, obtained by 10-day maceration at 15 °C 144 combinations = 8 base oils × 3 herbs (sage, thyme, basil) × 3 forms (IP, PP, L) × 2 weights (50 g and 100 g) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskoś, K.; Pisulewska, E.; Waligórski, P.; Janowiak, F.; Janeczko, A.; Sadura, I.; Polaszczyk, S.; Czyczyło-Mysza, I.M. Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils. Antioxidants 2021, 10, 781. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050781
Laskoś K, Pisulewska E, Waligórski P, Janowiak F, Janeczko A, Sadura I, Polaszczyk S, Czyczyło-Mysza IM. Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils. Antioxidants. 2021; 10(5):781. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050781
Chicago/Turabian StyleLaskoś, Kamila, Elżbieta Pisulewska, Piotr Waligórski, Franciszek Janowiak, Anna Janeczko, Iwona Sadura, Szymon Polaszczyk, and Ilona Mieczysława Czyczyło-Mysza. 2021. "Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils" Antioxidants 10, no. 5: 781. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10050781