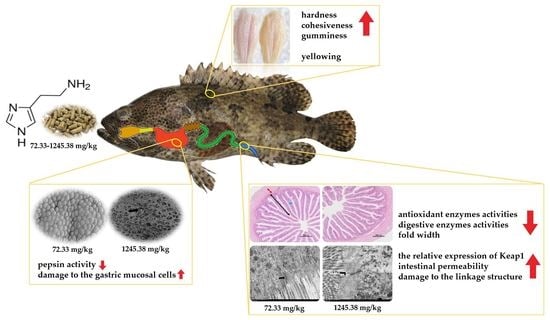
Dietary Histamine Impairs the Digestive Physiology Function and Muscle Quality of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Feed
2.2. Fish and Experimental Conditions
2.3. Sample Collection and Pre-Treatment
2.4. Chemical Composition Analysis
2.5. Biochemical Indexes Analyses
2.6. Histological Observation
2.7. Extraction of RNA and Real-Time Quantitative PCR Analysis
2.8. Muscle Texture and Color Analysis
2.9. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Digestive Enzyme Activity
3.3. Intestinal Permeability
3.4. Gastrointestinal Tract Structure
3.4.1. SEM of Gastric Mucosa Cell
3.4.2. Intestinal Morphology
3.4.3. TEM of Intestinal Mucosal Cell
3.5. Antioxidant Index
3.6. Dorsal Muscle Texture and Color
3.7. The Relative mRNA Expression of Tight Junction Proteins and Oxidative Stress-Related Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing Fishmeal and Fish Oil in Industrial Aquafeeds for Carnivorous Fish. Feed. Feed. Pract. Aquac. 2015, 203–233. [Google Scholar] [CrossRef]
- Ye, G.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, H.; Tan, B.; Zhang, S. A formulated diet improved digestive capacity, immune function and intestinal microbiota structure of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) when compared with chilled trash fish. Aquaculture 2020, 523, 735230. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Hu, L.; Yang, W.; Ai, C.; Sun, Y. Dose-dependent effects of histamine on growth, immunity and intestinal health in juvenile grouper (Epinephelus coioides). Front. Mar. Sci. 2021, 8, 685720. [Google Scholar] [CrossRef]
- Jaw, Y.-M.; Chen, Y.-Y.; Lee, Y.-C.; Lee, P.-H.; Jiang, C.-M.; Tsai, Y.-H. Histamine content and isolation of histamine-forming bacteria in fish meal and fish soluble concentrate. Fish. Sci. 2011, 78, 155–162. [Google Scholar] [CrossRef]
- Köse, S.; Quantick, P.; Hall, G. Changes in the levels of histamine during processing and storage of fish meal. Anim. Feed. Sci. Technol. 2003, 107, 161–172. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Fan, P.; Zhao, L.; Cheng, Y.; Wu, X.; Zeng, C. Survival, growth, sexual maturity and tissue histamine accumulation of the mysis, Neomysis awatschensis and N. japonica Nakazawa, fed histamine supplemented diets. Aquaculture 2010, 302, 256–260. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Liu, Z.; Yin, Y.; Xu, G.; Han, M.; Xie, L. Effect of dietary histamine on intestinal morphology, inflammatory status, and gut microbiota in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2021, 117, 95–103. [Google Scholar] [CrossRef]
- Shifrine, M.; Adler, H.E.; Ousterhout, L.E. The pathology of chicks fed histamine. Avian Dis. 1960, 4, 12–21. [Google Scholar] [CrossRef]
- Barnes, D.M.; Kirby, Y.K.; Oliver, K.G. Effects of Biogenic Amines on Growth and The Incidence of Proventricular Lesions in Broiler Chickens. Poult. Sci. 2001, 80, 906–911. [Google Scholar] [CrossRef]
- Zhai, S.; Wang, Y.; He, Y.; Chen, X. Oligomeric proanthocyanidins counteracts the negative effects of high level of dietary histamine on american eel (Anguilla rostrata). Front. Mar. Sci. 2020, 7, 549145. [Google Scholar] [CrossRef]
- He, J.; Wu, D.; Ye, Y.; Cai, C.; Wu, P.; Luo, Q.; Pu, Q. Effects of dietary histamine level on growth performance, serum biochemical indexes and gastrointestinal mucosa Structure of yellow catfish (Pelteobagrus fulvidraco). Chin. J. Anim. Nutr. 2018, 30, 2581–2593. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeuchi, T.; Satoh, S.; Toyama, K.; Okuzumi, M. Effect of dietary histidine or histamine on growth and development of stomach erosion in rainbow trout. Nippon. Suisan Gakkaishi 1987, 53, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Cai, P.; Zhai, S.; Chen, X. Effect of dietary histamine on growth performance, digestive enzyme activities and antioxidant indices in intestine of juvenile American eels (Anguilla rostrata). Feed Res. 2020, 2, 42–45. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, W.; Han, M.; Xu, G.; Xie, L.; Yin, Y.; Liang, J. The enterohepatic protection of pelteobagrus fulvidraco adding lactobacillus reuteri induced by histamine. Acta. Hydrobiol. Sin. 2019, 43, 94–101. [Google Scholar] [CrossRef]
- Shiozaki, K.; Nakano, T.; Yamaguchi, T.; Sato, M.; Sato, N. The protective effect of stevia extract on the gastric mucosa of rainbow trout Oncorhynchus mykiss (Walbaum) fed dietary histamine. Aquac. Res. 2004, 35, 1421–1428. [Google Scholar] [CrossRef]
- Opstvedt, J.; Mundheim, H.; Nygård, E.; Aase, H.; Pike, I.H. Reduced growth and feed consumption of Atlantic salmon (Salmo salar L.) fed fish meal made from stale fish is not due to increased content of biogenic amines. Aquaculture 2000, 188, 323–337. [Google Scholar] [CrossRef]
- Fan, X.; Qin, X.; Zhang, C.; Chen, J.; Zhu, Q. Nutritional and volatile flavor components of dorsal and ventral muscle from hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). J. GDOU 2018, 38, 39–46. [Google Scholar]
- Lu, Z.; Huang, H.; Huang, X.; Huang, W. Effects of hypoxic stress on antioxidant and energy metabolism of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus ♂). J. GDOU 2022, 42, 13–19. [Google Scholar] [CrossRef]
- Boonyaratpalin, M. Nutrient requirements of marine food fish cultured in Southeast Asia. Aquaculture 1997, 151, 283–313. [Google Scholar] [CrossRef]
- Zheng, C.; Cao, J.; Dong, X.; Chi, S.; Zhang, S.; Yang, Q.; Liu, H.; Deng, J.; Zhang, W.; Tan, B.; et al. Apparent digestibility coefficients of seven protein sources for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Acta Hydrobiol. Sin. 2022, 47, 1–12. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 1995. [Google Scholar]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish Shellfish. Immunol. 2021, 120, 497–506. [Google Scholar] [CrossRef]
- Chen, K.; Ye, Y.; Cai, C.; Wu, P.; Huang, Y.; Wu, T.; Lin, X.; Luo, Q.; Zhang, B.; Xiao, P.; et al. Effects of MDA on the growth performance, structure and function of hepatopancreas and intestine of grass carp (Ctenopharyngodon Idellus). Acta Hydrobiol. Sin. 2016, 40, 779–792. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, X.; Cheng, Y.; Yang, S. Effect of dietary histamine supplementation on growth, digestive enzyme activities and morphology of intestine and hepatopancreas in the Chinese mitten crab Eriocheir sinensis. SpringerPlus 2016, 5, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumsden, J.S.; Clark, P.; Hawthorn, S.; Minamikawa, M.; Fenwick, S.G.; Haycock, M.; Wybourne, B. Gastric dilation and air sacculitis in farmed chinook salmon, Oncorhynchus tshawytscha (Walbaum). J. Fish Dis. 2002, 25, 155–163. [Google Scholar] [CrossRef]
- Hu, J.; Ma, D.; Chen, X.; Zhai, S. Effect of dietary histamine on growth performance and muscle quality of American eel (Anguilla rostrata). Feed Res. 2019, 6, 34–37. [Google Scholar] [CrossRef]
- Reyes-Sosa, C.F.; Castellanos-Molina, R. Nutritional evaluation of gizzard erosion positive brown fish meal in starter diets for Nile tilapia, Oreochromis niloticus. Aquaculture 1995, 138, 323–329. [Google Scholar] [CrossRef]
- Hu, L.; Yun, B.; Xue, M.; Wang, J.; Wu, X.; Zheng, Y.; Han, F. Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus). Aquaculture 2013, 372, 52–61. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Cheng, W.; Li, W.; Chen, J.; Xie, L.; Xu, G. Effect of dietary histamine levels on growth performance and body pigmentation of Pelteobagrus Fulvidraco. FW Fish. 2017, 47, 79–84. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H. The impact of raw material freshness and processing temperature for fish meal on growth, feed efficiency and chemical composition of Atlantic halibut (Hippoglossus). Aquaculture 1997, 149, 87–106. [Google Scholar] [CrossRef]
- Yang, E.; Zhang, J.; Yang, L.; Amenyogbe, E.; Wang, W.; Huang, J.; Chen, G. Effects of hypoxia stress on digestive enzyme activities, intestinal structure and the expression of tight junction proteins coding genes in juvenile cobia (Rachycentron canadum). Aquac. Res. 2021, 52, 5630–5641. [Google Scholar] [CrossRef]
- Leng, X.; Wang, K.; Yang, F.; Duanmu, D.; Zhou, A. Effects of supplemental histamine on gastric acid secretion, digestive enzyme activities and intestinal microfloral of early weaned piglets. SAS 2003, 36, 324–328. [Google Scholar] [CrossRef]
- Kamiya, S.; Nagino, M.; Kanazawa, H.; Komatsu, S.; Mayumi, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Tanaka, R.; Nimura, Y. The value of bile replacement during external biliary drainage: An analysis of intestinal permeability, integrity, and microflora. Ann. Surg. 2004, 239, 510–517. [Google Scholar] [CrossRef]
- Geda, F.; Rekecki, A.; Decostere, A.; Bossier, P.; Wuyts, B.; Kalmar, I.; Janssens, G. Changes in intestinal morphology and amino acid catabolism in common carp at mildly elevated temperature as affected by dietary mannanoligosaccharides. Anim. Feed. Sci. Technol. 2012, 178, 95–102. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Simons, K.J. Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011, 128, 1139–1150.e4. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Zha, X.; Ma, Y.; Liu, X.; Elsabagh, M.; Wang, H.; Wang, M. Dietary L-Arginine or N-Carbamylglutamate Alleviates Colonic Barrier Injury, Oxidative Stress, and Inflammation by Modulation of Intestinal Microbiota in Intrauterine Growth-Retarded Suckling Lambs. Antioxidants 2022, 11, 2251. [Google Scholar] [CrossRef]
- Gu, M.; Jia, Q.; Zhang, Z.; Bai, N.; Xu, X.; Xu, B. Soya-saponins induce intestinal inflammation and barrier dysfunction in juvenile turbot (Scophthalmus maximus). Fish Shellfish. Immunol. 2018, 77, 264–272. [Google Scholar] [CrossRef]
- Fukudome, I.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Baba, R.; Kumagai, N.; Oba, K.; Fujita, M.; et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2013, 47, 100–107. [Google Scholar] [CrossRef]
- Qiao, X.; Wu, H. Detection of D-lactic acid and endotoxin and effect of dietary fiber complex on intestinal mucosal barrier in rats with portal hypertension. CHN Med. Herald 2014, 11, 26–32. [Google Scholar]
- Ding, L.-A.; Li, J.-S.; Li, Y.-S.; Zhu, N.-T.; Liu, F.-N.; Tan, L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J. Gastroenterol. 2004, 10, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E.A.; Mu, D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.-P.; Li, Y.; Hou, Y.-M.; Qiu, H.; Zhou, Q.-C. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac. Res. 2015, 48, 149–160. [Google Scholar] [CrossRef]
- Denning, T.L.; Takaishi, H.; Crowe, S.E.; Boldogh, I.; Jevnikar, A.; Ernst, P.B. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free. Radic. Biol. Med. 2002, 33, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Rashidian, G.; Hoseinifar, S.H.; Naserabad, S.S.; Van Doan, H. Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish. Immunol. 2020, 99, 267–273. [Google Scholar] [CrossRef]
- Hu, B.; Song, L.; Mao, S.; Xu, P. Effects of four chinese herbal preparations on growth performance and antioxidant activity in juvenile Micropterus salmoides. J. GDOU 2019, 39, 101–107. [Google Scholar] [CrossRef]
- An, W.; He, H.; Dong, X.; Tan, B.; Yang, Q.; Chi, S.; Zhang, S.; Liu, H.; Yang, Y. Regulation of growth, fatty acid profiles, hematological characteristics and hepatopancreatic histology by different dietary n-3 highly unsaturated fatty acids levels in the first stages of juvenile Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 17, 100321. [Google Scholar] [CrossRef]
- Kwiecień, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish. Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, J.; Duan, Y.-F.; Niu, J.; Wang, J.; Huang, Z.; Lin, H.-Z. Effects of dietary chlorogenic acid on growth performance, antioxidant capacity of white shrimp Litopenaeus vannamei under normal condition and combined stress of low-salinity and nitrite. Fish Shellfish. Immunol. 2015, 43, 337–345. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Zhou, C.; Huang, Z.; Tan, L.; Xun, P.; Huang, Q.; Lin, H.; Ye, C.; Wang, A. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish. Immunol. 2017, 73, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K. Intrauterine growth retardation affects intestinal health of suckling piglets via altering intestinal antioxidant capacity, glucose uptake, tight junction, and immune responses. Oxidative Med. Cell 2022, 2022, 2644205. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.-T.; Amenyogbe, E.; Chen, G.; Huang, J.-S. Effects of feed fat level on growth performance, body composition and serum biochemical indices of hybrid grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 2020, 530, 735813. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.; Li, X.; Dong, Y.; Wang, X.; Mu, W.; Gatlin, D.M.; Zhang, Y. The optimum dietary isoleucine requirement of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquac. Nutr. 2020, 26, 1295–1310. [Google Scholar] [CrossRef]
- Zou, C.; Xu, M.; Chen, L.; Liu, Q.; Zhou, Y.; Sun, Z.; Ye, H.; Su, N.; Ye, C.; Wang, A. Xiaochaihu Decoction reduces hepatic steatosis and improves D-GalN/LPS-induced liver injury in hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Fish Shellfish. Immunol. 2019, 91, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Pyun, B.-J.; Jo, K.; Lee, J.Y.; Lee, A.; Jung, M.-A.; Hwang, Y.-H.; Jung, D.H.; Ji, K.-Y.; Choi, S.; Kim, Y.H.; et al. Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells. Antioxidants 2022, 11, 2256. [Google Scholar] [CrossRef]
- Zhong, X.; Li, X.; Cai, W.; Xu, C.; Li, Q.; Huang, J.; Liu, W. Effects of fermented feed on growth performance, digestive enzyme activity, fillet quality and immunity of juvenile carp (Cyprinus carpio). J. NJAU 2018, 41, 154–162. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Zhi, X.; Zhao, X.; Wang, G.; Chi, S.; Tan, B. Effects of replacing fish meal with enzyme-digested poultry by-product meal on muscle quality and expression of muscle growth-related factors of hybrid grouper (Epinephelus fuscoguttatus ♀ × E.lanceolatus ♂). Chin. J. Anim. Nutr. 2021, 33, 6999–7011. [Google Scholar] [CrossRef]
- Wu, F.; Wen, H.; Jiang, M.; Liu, W.; Tian, J.; Yang, C.; Huang, F. Effect of dietary vitamin C on growth performance, flesh quality and antioxidant function in genetically improved farmed tilapia. J. Fish Sci. CHN 2015, 22, 79–87. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, Y.; Zhou, X.; Yin, L.; Feng, L.; Jiang, W.; Liu, Y.; Zhao, Y. Effects of replacement ratio of fish meal by soybean meal in extruded diets on muscle quality of jian carp (Cyprinus carpio var. Jian). Chin. J. Anim. Nutr. 2015, 27, 623–630. [Google Scholar] [CrossRef]
- Greene, B.E.; Price, L.G. Oxidation-induced color and flavor changes in meat. J. Agric. Food Chem. 1975, 23, 164–167. [Google Scholar] [CrossRef]





| Ingredients (%) | Diets | ||||||
|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | |
| Fish meal | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 |
| Soy protein concentrate | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 |
| Soybean meal | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Wheat flour | 17.91 | 17.91 | 17.91 | 17.91 | 17.91 | 17.91 | 17.91 |
| Soybean oil | 5.30 | 5.30 | 5.30 | 5.30 | 5.30 | 5.30 | 5.30 |
| Soybean lecithin | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Ca(H2PO4)2 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Choline chloride | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin C | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Vitamin and mineral premixes 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Histamine dihydrochloride (mg/kg) 2 | 0.00 | 50.70 | 101.39 | 202.78 | 405.56 | 811.13 | 1622.25 |
| Cellulose microcrystalline (mg/kg) | 1622.25 | 1571.55 | 1520.86 | 1419.47 | 1216.69 | 811.12 | 0.00 |
| Proximate composition | |||||||
| Dry matter (DM, %) | 92.34 | 92.28 | 92.30 | 92.30 | 92.35 | 92.40 | 92.36 |
| Crude protein (% DM) | 49.50 | 50.20 | 48.97 | 49.18 | 49.23 | 50.11 | 49.79 |
| Crude lipid (% DM) | 11.27 | 11.30 | 11.28 | 11.28 | 11.28 | 11.27 | 11.26 |
| Histamine (mg/kg DM) | 72.33 | 99.56 | 138.60 | 225.35 | 404.12 | 662.12 | 1245.38 |
| Target Gene | Primer Sequence | GenBank Accession No. |
|---|---|---|
| claudin3 | F-AGCCTTCATCGGCAGCAA R-GGATGCCTCGTCGTCAATG | EU714179.1 |
| occludin | F-GGAGGAGAAACAGGGAATGAACT R-TCTGCTACAGCCTGGTATTTGG | KF861990.1 |
| Keap1 1 | F-TCCACAAACCCACCAAAGTAA R-TCCACCAACAGCGTAGAAAAG | XM_018665037.1 |
| Nrf2 2 | F-TATGGAGATGGGTCCTTTGGTG R-GCTTCTTTTCCTGCGTCTGTTG | KU892416.1 |
| β-Actin | F-GGCTACTCCTTCACCACCACA R-TCTGGGCAACGGAACCTCT | AY510710.2 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| Initial body weight (g) | 14.77 ± 0.01 | 14.80 ± 0.01 | 14.76 ± 0.01 | 14.81 ± 0.00 | 14.76 ± 0.05 | 14.76 ± 0.01 | 14.77 ± 0.01 | 0.486 | 0.581 |
| Final body weight (g) | 116.39 ± 0.25 | 117.19 ± 1.49 | 115.01 ± 1.84 | 114.62 ± 1.03 | 114.76 ± 1.53 | 114.18 ± 0.46 | 113.57 ± 0.70 | 0.080 | 0.159 |
| Weight gain | 6.88 ± 0.03 | 6.81 ± 0.02 | 6.79 ± 0.13 | 6.74 ± 0.07 | 6.78 ± 0.09 | 6.73 ± 0.03 | 6.69 ± 0.05 | 0.128 | 0.285 |
| Specific growth ratio (%/d) | 3.62 ± 0.01 | 3.60 ± 0.00 | 3.60 ± 0.03 | 3.59 ± 0.01 | 3.60 ± 0.02 | 3.59 ± 0.01 | 3.58 ± 0.01 | 0.131 | 0.291 |
| Voluntary feed intake (mg/MBW/d) | 23.62 ± 0.50 | 23.26 ± 0.23 | 23.47 ± 0.42 | 23.31 ± 0.46 | 23.86 ± 0.15 | 23.61 ± 0.09 | 23.73 ± 0.39 | 0.414 | 0.634 |
| Feed conversion ratio | 0.83 ± 0.01 | 0.87 ± 0.01 | 0.87 ± 0.04 | 0.89 ± 0.02 | 0.88 ± 0.03 | 0.89 ± 0.02 | 0.90 ± 0.02 | 0.059 | 0.110 |
| Protein efficiency ratio | 2.44 ± 0.03 | 2.38 ± 0.04 | 2.40 ± 0.07 | 2.37 ± 0.03 | 2.31 ± 0.03 | 2.32 ± 0.07 | 2.29 ± 0.02 | 0.021 | 0.041 |
| Survival rate (%) | 96.67 ± 0.00 | 97.78 ± 1.11 | 96.67 ± 3.33 | 93.33 ± 1.93 | 91.11 ± 2.94 | 90.00 ± 3.33 | 90.00 ± 3.33 | 0.007 | 0.003 |
| Hepatosomatic index (%) | 4.55 ± 0.12 | 4.46 ± 0.29 | 4.20 ± 0.22 | 4.09 ± 0.18 | 4.02 ± 0.29 | 3.94 ± 0.22 | 3.87 ± 0.25 | 0.043 | 0.056 |
| Viscerosomatic index (%) | 12.79 ± 0.35 | 12.51 ± 0.61 | 12.16 ± 0.32 | 11.97 ± 0.46 | 11.86 ± 0.31 | 11.67 ± 0.42 | 11.50 ± 0.49 | 0.041 | 0.061 |
| Condition factor (g/cm3) | 3.01 ± 0.09 | 2.86 ± 0.12 | 2.82 ± 0.08 | 2.87 ± 0.10 | 2.74 ± 0.07 | 2.73 ± 0.05 | 2.73 ± 0.03 | 0.023 | 0.011 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| Stomach | |||||||||
| Pepsin (U/mg protein) | 56.05 ± 2.82 bc | 58.39 ± 1.36 c | 51.32 ± 4.41 abc | 49.60 ± 3.32 abc | 45.57 ± 3.18 ab | 44.56 ± 3.72 ab | 42.57 ± 1.70 a | 0.009 | 0.008 |
| Foregut | |||||||||
| Trypsin (U/μg protein) | 0.25 ± 0.02 c | 0.25 ± 0.02 c | 0.22 ± 0.01 bc | 0.19 ± 0.01 ab | 0.19 ± 0.01 ab | 0.17 ± 0.01 a | 0.18 ± 0.01 ab | 0.002 | <0.001 |
| Lipase (U/g protein) | 1.65 ± 0.04 c | 1.68 ± 0.06 c | 1.49 ± 0.06 abc | 1.54 ± 0.15 bc | 1.37 ± 0.07 ab | 1.26 ± 0.03 a | 1.27 ± 0.09 a | <0.001 | <0.001 |
| Amylase (U/mg protein) | 0.38 ± 0.00 c | 0.33 ± 0.02 bc | 0.31 ± 0.02 abc | 0.26 ± 0.02 ab | 0.29 ± 0.00 ab | 0.25 ± 0.03 ab | 0.25 ± 0.05 a | 0.009 | 0.005 |
| Maltase (U/mg protein) | 13.93 ± 1.80 | 13.30 ± 0.56 | 12.88 ± 0.52 | 12.58 ± 1.49 | 11.49 ± 1.56 | 10.83 ± 0.74 | 10.72 ± 1.51 | 0.077 | 0.112 |
| Na+/K+-ATPase (U/mg protein) | 1.64 ± 0.12 c | 1.63 ± 0.09 c | 1.48 ± 0.06 bc | 1.54 ± 0.08 bc | 1.46 ± 0.09 bc | 1.31 ± 0.03 ab | 1.14 ± 0.02 a | <0.001 | <0.001 |
| Ca2+/Mg2+-ATPase (U/mg protein) | 5.71 ± 0.49 | 5.73 ± 0.87 | 5.63 ± 0.43 | 5.52 ± 0.08 | 5.53 ± 0.74 | 5.12 ± 0.78 | 4.96 ± 0.07 | 0.213 | 0.450 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| Fold height (μm) | 643.16 ± 19.54 | 646.77 ± 17.25 | 647.34 ± 12.29 | 645.80 ± 19.92 | 637.39 ± 12.97 | 627.73 ± 7.95 | 617.98 ± 15.18 | 0.167 | 0.382 |
| Fold width (μm) | 82.28 ± 2.41 b | 79.04 ± 3.21 ab | 80.85 ± 3.14 ab | 80.48 ± 2.77 ab | 76.38 ± 3.16 ab | 73.16 ± 3.05 ab | 69.51 ± 2.63 a | 0.008 | 0.025 |
| Muscular thickness (μm) | 147.27 ± 7.00 | 144.93 ± 9.01 | 145.42 ± 8.22 | 154.83 ± 6.98 | 155.31 ± 6.42 | 138.20 ± 6.71 | 142.61 ± 10.33 | 0.531 | 0.823 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| SOD (U/mL) | 62.85 ± 2.26 c | 61.64 ± 1.38 bc | 56.76 ± 1.81 abc | 56.70 ± 3.38 abc | 54.90 ± 1.50 ab | 55.35 ± 2.13 a | 52.83 ± 1.65 a | 0.003 | 0.002 |
| CAT (U/mL) | 9.71 ± 1.36 b | 8.75 ± 1.19 ab | 7.77 ± 0.37 ab | 7.23 ± 0.52 ab | 7.15 ± 0.79 ab | 6.72 ± 1.04 a | 6.10 ± 0.59 a | 0.009 | 0.017 |
| POD (U/mL) | 0.34 ± 0.01 b | 0.32 ± 0.02 ab | 0.30 ± 0.03 ab | 0.29 ± 0.01 ab | 0.30 ± 0.01 ab | 0.28 ± 0.02 ab | 0.26 ± 0.02 a | 0.004 | 0.014 |
| GPx (U/mL) | 0.17 ± 0.01 b | 0.15 ± 0.01 ab | 0.15 ± 0.01 ab | 0.15 ± 0.01 ab | 0.14 ± 0.00 a | 0.13 ± 0.00 a | 0.13 ± 0.01 a | 0.012 | 0.005 |
| GR (U/mL) | 0.15 ± 0.01 b | 0.15 ± 0.01 b | 0.13 ± 0.01 b | 0.13 ± 0.01 b | 0.12 ± 0.00 ab | 0.10 ± 0.00 a | 0.10 ± 0.01 a | <0.001 | <0.001 |
| TAC (nmol/L) | 1.83 ± 0.05 c | 1.72 ± 0.05 bc | 1.62 ± 0.03 ab | 1.62 ± 0.00 ab | 1.59 ± 0.04 ab | 1.55 ± 0.06 a | 1.53 ± 0.07 a | 0.002 | 0.001 |
| MDA (nmol/L) | 3.30 ± 0.43 a | 3.08 ± 0.24 a | 3.27 ± 0.22 a | 4.95 ± 0.44 b | 6.03 ± 0.71 b | 6.48 ± 0.47 b | 6.43 ± 0.58 b | <0.001 | <0.001 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| SOD (U/μg protein) | 0.60 ± 0.01 | 0.58 ± 0.02 | 0.59 ± 0.02 | 0.56 ± 0.02 | 0.62 ± 0.02 | 0.61 ± 0.01 | 0.60 ± 0.03 | 0.284 | 0.315 |
| CAT (U/mg protein) | 17.46 ± 0.91 b | 17.51 ± 0.73 b | 16.82 ± 1.84 b | 16.85 ± 3.11 b | 15.85 ± 1.07 ab | 16.61 ± 0.85 ab | 13.21 ± 0.50 a | 0.047 | 0.139 |
| POD (U/g protein) | 27.13 ± 3.02 | 27.08 ± 0.57 | 25.57 ± 2.39 | 23.98 ± 3.43 | 22.16 ± 2.29 | 20.05 ± 3.16 | 18.69 ± 1.13 | 0.012 | 0.021 |
| GPx (U/mg protein) | 15.27 ± 1.31 c | 16.08 ± 0.46 c | 13.46 ± 0.57 bc | 12.79 ± 0.97 abc | 11.34 ± 0.84 ab | 9.57 ± 1.22 a | 9.64 ± 1.65 a | 0.001 | <0.001 |
| GR (U/mg protein) | 2.49 ± 0.21 c | 2.62 ± 0.28 c | 2.31 ± 0.09 bc | 2.22 ± 0.15 bc | 1.77 ± 0.11 ab | 1.52 ± 0.21 a | 1.67 ± 0.16 a | 0.003 | <0.001 |
| TAC (mmol/g protein) | 15.00 ± 2.64 b | 13.66 ± 1.46 ab | 13.07 ± 1.79 ab | 12.97 ± 1.14 ab | 10.57 ± 0.98 ab | 10.32 ± 2.07 ab | 8.75 ± 1.29 a | 0.007 | 0.014 |
| MDA (nmol/g protein) | 22.87 ± 5.92 a | 26.77 ± 5.08 ab | 27.63 ± 3.34 ab | 28.26 ± 2.36 ab | 29.00 ± 2.61 ab | 34.61 ± 6.42 ab | 39.31 ± 2.50 b | 0.001 | 0.004 |
| Diets | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| Hardness (kg) | 0.95 ± 0.07 a | 0.96 ± 0.08 a | 1.06 ± 0.05 ab | 1.08 ± 0.09 ab | 1.16 ± 0.08 ab | 1.15 ± 0.09 ab | 1.25 ± 0.08 b | 0.003 | 0.008 |
| Adhesiveness (g.sec) | −11.29 ± 2.74 | −9.13 ± 1.22 | −10.33 ± 1.91 | −8.35 ± 1.00 | −11.03 ± 2.14 | −10.97 ± 2.29 | −10.50 ± 2.06 | 0.729 | 0.922 |
| Springiness | 0.23 ± 0.02 | 0.26 ± 0.06 | 0.22 ± 0.02 | 0.28 ± 0.04 | 0.23 ± 0.03 | 0.23 ± 0.04 | 0.24 ± 0.05 | 0.817 | 0.961 |
| Cohesiveness | 0.10 ± 0.00 a | 0.09 ± 0.00 a | 0.10 ± 0.01 a | 0.12 ± 0.00 ab | 0.11 ± 0.01 ab | 0.13 ± 0.01 bc | 0.15 ± 0.01 c | <0.001 | <0.001 |
| Gumminess | 85.85 ± 13.39 a | 86.86 ± 7.46 a | 110.87 ± 13.44 ab | 115.02 ± 13.15 ab | 122.43 ± 3.75 ab | 118.84 ± 11.89 b | 113.67 ± 23.77 b | 0.003 | 0.005 |
| Chewiness | 20.75 ± 0.75 a | 19.94 ± 3.04 a | 27.83 ± 4.67 ab | 38.18 ± 5.83 ab | 36.23 ± 7.67 ab | 39.63 ± 8.51 ab | 42.88 ± 7.39 b | 0.008 | 0.008 |
| Resilience | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.123 | 0.262 |
| Diets | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H0 | H3 | H6 | H12 | H24 | H48 | H96 | Linear | Quadratic | |
| L value 1 | 59.52 ± 1.40 | 59.76 ± 1.43 | 59.71 ± 0.94 | 60.08 ± 0.84 | 60.10 ± 0.70 | 60.19 ± 1.73 | 59.34 ± 1.49 | 0.906 | 0.832 |
| a value 2 | −3.94 ± 0.13 | −3.63 ± 0.32 | −3.19 ± 0.46 | −3.75 ± 0.33 | −3.87 ± 0.24 | −3.92 ± 0.12 | −3.72 ± 0.31 | 0.710 | 0.840 |
| b value 3 | 6.43 ± 0.29 a | 7.54 ± 0.70 ab | 7.59 ± 0.75 ab | 6.93 ± 0.36 ab | 7.77 ± 0.39 ab | 8.42 ± 0.28 b | 8.48 ± 0.28 b | 0.010 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, H.; Liu, Y.; Zhu, L.; Fan, J.; Huang, H.; Jiang, W.; Deng, J.; Tan, B. Dietary Histamine Impairs the Digestive Physiology Function and Muscle Quality of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Antioxidants 2023, 12, 502. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020502
Zhang Y, Zhou H, Liu Y, Zhu L, Fan J, Huang H, Jiang W, Deng J, Tan B. Dietary Histamine Impairs the Digestive Physiology Function and Muscle Quality of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Antioxidants. 2023; 12(2):502. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020502
Chicago/Turabian StyleZhang, Yumeng, Hang Zhou, Yu Liu, Lulu Zhu, Jiongting Fan, Huajing Huang, Wen Jiang, Junming Deng, and Beiping Tan. 2023. "Dietary Histamine Impairs the Digestive Physiology Function and Muscle Quality of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂)" Antioxidants 12, no. 2: 502. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020502