Valganciclovir—Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Valganciclovir and Ganciclovir Use and Their Adequacy
3.3. Valganciclovir and Ganciclovir Serum Levels and Their Variability
3.4. Risk Factors, Valganciclovir and Ganciclovir Serum Levels and Association with Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef]
- Griffiths, P.D. Prophylaxis against CMV infection in transplant patients. J. Antimicrob. Chemother. 1997, 39, 299–301. [Google Scholar] [CrossRef] [Green Version]
- McGavin, J.K.; Goa, K.L. Ganciclovir: An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 2001, 61, 1153–1183. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Kitzberger, R.; Drolz, A.; Zauner, C.; Jager, W.; Bohmdorfer, M.; Kraff, S.; Fritsch, A.; Thalhammer, F.; Fuhrmann, V.; et al. Pharmacokinetics of ganciclovir during continuous venovenous hemodiafiltration in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, R.; Singh, N. Cytomegalovirus infection in critically ill patients: A systematic review. Crit. Care 2009, 13, R68. [Google Scholar] [CrossRef] [Green Version]
- Czock, D.; Scholle, C.; Rasche, F.M.; Schaarschmidt, D.; Keller, F. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin. Pharm. 2002, 72, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Heel, R.C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 1990, 39, 597–638. [Google Scholar] [CrossRef]
- Nevins, T.E.; Dunn, D.L. Use of ganciclovir for cytomegalovirus infection. J. Am. Soc. Nephrol. 1992, 2, S270–S273. [Google Scholar] [PubMed]
- Roche Laboratories, Inc. Cymevene (Ganciclovir) Package Insert; Roche Laboratories, Inc.: Basel, Switzerland, 2009. [Google Scholar]
- Vezina, H.E.; Brundage, R.C.; Balfour, H.H., Jr. Population pharmacokinetics of valganciclovir prophylaxis in paediatric and adult solid organ transplant recipients. Br. J. Clin. Pharm. 2014, 78, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Perrottet, N.; Manuel, O.; Lamoth, F.; Venetz, J.P.; Sahli, R.; Decosterd, L.A.; Buclin, T.; Pascual, M.; Meylan, P. Variable viral clearance despite adequate ganciclovir plasma levels during valganciclovir treatment for cytomegalovirus disease in D+/R- transplant recipients. BMC Infect. Dis 2010, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, B.M.; Barreto, J.N.; Frazee, E.; Crow, S.A.; Dierkhising, R.A.; Jannetto, P.J.; Tosh, P.K.; Razonable, R.R. Relationship of Ganciclovir Therapeutic Drug Monitoring with Clinical Efficacy and Patient Safety. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrottet, N.; Csajka, C.; Pascual, M.; Manuel, O.; Lamoth, F.; Meylan, P.; Aubert, J.D.; Venetz, J.P.; Soccal, P.; Decosterd, L.A.; et al. Population pharmacokinetics of ganciclovir in solid-organ transplant recipients receiving oral valganciclovir. Antimicrob. Agents Chemother. 2009, 53, 3017–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vethamuthu, J.; Feber, J.; Chretien, A.; Lampe, D.; Filler, G. Unexpectedly high inter- and intrapatient variability of ganciclovir levels in children. Pediatr. Transpl. 2007, 11, 301–305. [Google Scholar] [CrossRef]
- Gimenez, E.; Solano, C.; Azanza, J.R.; Amat, P.; Navarro, D. Monitoring of trough plasma ganciclovir levels and peripheral blood cytomegalovirus (CMV)-specific CD8+ T cells to predict CMV DNAemia clearance in preemptively treated allogeneic stem cell transplant recipients. Antimicrob. Agents Chemother. 2014, 58, 5602–5605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.C.; Partovi, N.; Ensom, M.H. Ganciclovir in solid organ transplant recipients: Is there a role for clinical pharmacokinetic monitoring? Drug Monit. 2004, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.A.; Slavin, M.; Roberts, J.A.; Yong, M. Optimization of Ganciclovir use in allogeneic hematopoietic cell transplant recipients—The role of therapeutic drug monitoring. Expert Rev. Anti. Infect. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Rodriguez-Gonzalez, C.G.; Munoz, P.; Caliz, B.; Sanjurjo, M.; Bouza, E.; Group, C.S. Evaluation of antifungal use in a tertiary care institution: Antifungal stewardship urgently needed. J. Antimicrob. Chemother. 2014, 69, 1993–1999. [Google Scholar] [CrossRef] [Green Version]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Asberg, A.; Chou, S.; Danziger-Isakov, L.; Humar, A.; Transplantation Society International, C.M.V.C.G. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013, 96, 333–360. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Kodama, T.; Noguchi, Y.; Deguchi, K.; Nomura, M.; Saka, R.; Watanabe, M.; Tazuke, Y.; Bessho, K.; Okuyama, H. One Year of Preemptive Valganciclovir Administration in Children After Liver Transplantation. Transpl. Proc. 2020, 52, 1852–1854. [Google Scholar] [CrossRef]
- Peled, O.; Berkovitch, M.; Rom, E.; Bilavsky, E.; Bernfeld, Y.; Dorfman, L.; Pappo, A.; Ziv-Baran, T.; Brandriss, N.; Bar-Haim, A.; et al. Valganciclovir Dosing for Cytomegalovirus Prophylaxis in Pediatric Solid-organ Transplant Recipients: A Prospective Pharmacokinetic Study. Pediatr. Infect. Dis. J. 2017, 36, 745–750. [Google Scholar] [CrossRef]
- Padulles, A.; Colom, H.; Armendariz, Y.; Cerezo, G.; Caldes, A.; Pou, L.; Torras, J.; Grinyo, J.M.; Lloberas, N. Determination of ganciclovir in human plasma by ultra performance liquid chromatography-UV detection. Clin. Biochem. 2012, 45, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D.; et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrottet, N.; Decosterd, L.A.; Meylan, P.; Pascual, M.; Biollaz, J.; Buclin, T. Valganciclovir in adult solid organ transplant recipients: Pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin. Pharm. 2009, 48, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Chamberlain, A.; Tang, P.; Turpin, B.; Danziger-Isakov, L. Paediatric ganciclovir dosing in extracorporeal membrane oxygenation: Is standard dosing good enough? J. Clin. Pharm. 2019, 45, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Royakkers, A.A.N.M.; Schultz, M.J.; Spronk, P.E. Cystatin C as a Marker of Renal Function in Critically Ill Patients at Risk for or with Acute Renal Failure. Intensive Care Med. 2007, 583–591. [Google Scholar] [CrossRef]
- Palacio-Lacambra, M.E.; Comas-Reixach, I.; Blanco-Grau, A.; Suñé-Negre, J.M.; Segarra-Medrano, A.; Montoro-Ronsano, J.B. Comparison of the Cockcroft-Gault, MDRD and CKD-EPI equations for estimating ganciclovir clearance. Br. J. Clin. Pharm. 2018, 84, 2120–2128. [Google Scholar] [CrossRef] [Green Version]
- Erice, A.; Jordan, M.C.; Chace, B.A.; Fletcher, C.; Chinnock, B.J.; Balfour, H.H., Jr. Ganciclovir treatment of cytomegalovirus disease in transplant recipients and other immunocompromised hosts. JAMA 1987, 257, 3082–3087. [Google Scholar] [CrossRef]
- Piketty, C.; Bardin, C.; Gilquin, J.; Mahe, V.; Kazatchkine, M.D.; Chast, F. Low plasma concentrations achieved with conventional schedules of administration of ganciclovir in patients with AIDS. J. Infect. Dis 1996, 174, 188–190. [Google Scholar] [CrossRef]
- Fishman, J.A.; Doran, M.T.; Volpicelli, S.A.; Cosimi, A.B.; Flood, J.G.; Rubin, R.H. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation 2000, 69, 389–394. [Google Scholar] [CrossRef]
- Fletcher, C.V.; Beatty, C.C.; Balfour, H.H. Ganciclovir disposition in patients with renal insufficiency-implications for dose adjustment. Pharmacotherapy 1991, 11, 277. [Google Scholar]
- Martson, A.G.; Touw, D.; Damman, K.; Bakker, M.; Lansink-Hartgring, A.O.; van der Werf, T.; Knoester, M.; Alffenaar, J.C. Ganciclovir Therapeutic Drug Monitoring: A Case Series. Drug Monit. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, F.; Pou, L.; Pascual, C. Serum monitoring of ganciclovir (abstract). Ther. Drug Monit. 1993, 15, 156. [Google Scholar] [CrossRef]
- Fletcher, C.; Sawchuk, R.; Chinnock, B.; de Miranda, P.; Balfour, H.H., Jr. Human pharmacokinetics of the antiviral drug DHPG. Clin. Pharm. 1986, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Piketty, C.; Bardin, C.; Gilquin, J.; Gairard, A.; Kazatchkine, M.D.; Chast, F. Monitoring plasma levels of ganciclovir in AIDS patients receiving oral ganciclovir as maintenance therapy for CMV retinitis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2000, 6, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotkin, S.A.; Drew, W.L.; Felsenstein, D.; Hirsch, M.S. Sensitivity of clinical isolates of human cytomegalovirus to 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J. Infect. Dis. 1985, 152, 833–834. [Google Scholar] [CrossRef]
- Landry, M.L.; Stanat, S.; Biron, K.; Brambilla, D.; Britt, W.; Jokela, J.; Chou, S.; Drew, W.L.; Erice, A.; Gilliam, B.; et al. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob. Agents Chemother. 2000, 44, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, H.; Paya, C.V.; Pescovitz, M.D.; Humar, A.; Dominguez, E.; Washburn, K.; Blumberg, E.; Alexander, B.; Freeman, R.; Heaton, N.; et al. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 2005, 79, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Manuel, O.; Venetz, J.P.; Fellay, J.; Wasserfallen, J.B.; Sturzenegger, N.; Fontana, M.; Matter, M.; Meylan, P.R.; Pascual, M. Efficacy and safety of universal valganciclovir prophylaxis combined with a tacrolimus/mycophenolate-based regimen in kidney transplantation. Swiss Med. Wkly. 2007, 137, 669–676. [Google Scholar]
- Shepp, D.H.; Dandliker, P.S.; de Miranda, P.; Burnette, T.C.; Cederberg, D.M.; Kirk, L.E.; Meyers, J.D. Activity of 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine in the treatment of cytomegalovirus pneumonia. Ann. Intern. Med. 1985, 3, 368–373. [Google Scholar] [CrossRef]
- Chen, B.; Hu, S.S.; Rui, W.B.; An, H.M.; Zhai, X.H.; Wang, X.H.; Lu, J.Q.; Shao, K.; Zhou, P.J. Population Pharmacokinetics and Bayesian Estimation of the Area Under the Concentration-Time Curve for Ganciclovir in Adult Chinese Renal Allograft Recipients After Valganciclovir Administration. J. Clin. Pharm. 2020. [Google Scholar] [CrossRef]
- Cintra-Cabrera, M.; Suarez-Benjumea, A.; Bernal-Blanco, G.; Gonzalez-Roncero, F.M.; Toapanta-Gaibor, N.G.; Suner-Poblet, M.; Perez-Valdivia, M.A.; Fernandez-Cuenca, F.; Gentil-Govantes, M.A.; Rocha-Castilla, J.L. Resistant Cytomegalovirus Infection After Renal Transplantation: Literature Review. Transpl. Proc. 2018, 50, 575–577. [Google Scholar] [CrossRef] [PubMed]



| GLOBAL | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Global (n = 70) | Poor Clinical Outcome (n = 31) | Favorable Clinical Outcome (n = 39) | p Value | OR | 95 % CI | p Value |
| General characteristics | |||||||
| Age (years), median (IQR) | 59.2 (46.5–69.7) | 64.9 (52.8–72.6) | 53.5 (42.4–65.8) | 0.046 | |||
| Sex, male, n (%) | 52 (74.3) | 22 (71) | 30 (76.9) | 0.594 | |||
| Weight (kg), median (IQR) | 65.8 (56.0–75.12) | 68.6 (57.6–78.6) | 65 (55–75) | 0.493 | |||
| Body mass index (mg/kg2), median (IQR) | 24.0 (20.6–27.7) | 25.7 (22.8–28.7) | 23 (19.4–26.8) | 0.105 | |||
| Race, n (%) | 1.0 | ||||||
| Caucasian | 60 (85.7) | 27 (87.1) | 33 (84.6) | ||||
| Other | 10 (14.3) | 4 (12.9) | 6 (15.4) | ||||
| Department of admission, n (%) | 0.163 | ||||||
| Medical | 47 (67.1) | 18 (58.1) | 29 (74.4) | ||||
| Surgical | 8 (11.4) | 3 (9.7) | 5 (12.8) | ||||
| ICU | 15 (21.4) | 10 (32.3) | 5 (12.8) | ||||
| Underlying disease, n (%) | |||||||
| Cardiac disease | 21 (30.0) | 12 (38.7) | 9 (23.1) | 0.194 | |||
| Diabetes mellitus | 20 (28.6) | 13 (41.9) | 7 (17.9) | 0.029 | 4.173 | 1.147–15.179 | 0.030 |
| Solid tumor | 8 (11.4) | 4 (12.9) | 4 (10.3) | 1.0 | |||
| Chronic renal failure | 20 (28.6) | 11 (35.5) | 9 (23.1) | 0.295 | |||
| Liver disease | 13 (18.6) | 7 (22.6) | 6 (15.4) | 0.541 | |||
| HIV infection | 8 (11.4) | 3 (9.7) | 5 (12.8) | 0.726 | |||
| Neurologic disease | 7 (10.0) | 2 (6.5) | 5 (12.8) | 0.452 | |||
| Chronic Obstructive Pulmonary Disease | 5 (7.1) | 3 (9.7) | 2 (5.1) | 0.649 | |||
| Solid Organ Transplantation | 26 (37.1) | 12 (38.7) | 14 (35.9) | 1.0 | |||
| Cardiac transplant | 10 (14.3) | 5 (16.1) | 5 (12.8) | 0.741 | |||
| Liver transplant | 11 (15.7) | 6 (19.4) | 5 (12.8) | 0.520 | |||
| Renal transplant * | 6 (8.6) | 2 (6.5) | 4 (10.3) | 0.687 | |||
| Haematologic neoplasia | 7 (10.0) | 2 (6.5) | 5 (12.8) | 0.452 | |||
| Psychiatric disease | 5 (7.1) | 2 (6.5) | 3 (7.7) | 1.0 | |||
| Other | 3 (4.3) | 2 (6.5) | 1 (2.6) | 0.580 | |||
| Charlson’s index, median (IQR) | 3 (1.7–5.0) | 4 (3-6) | 3 (1–5) | 0.098 | |||
| McCabe index, n (%) | 0.408 | ||||||
| Non-fatal | 40 (57.1) | 16 (51.6) | 24 (61.5) | ||||
| Ultimately fatal | 21 (30.0) | 9 (29.0) | 12 (30.8) | ||||
| Rapidly fatal | 9 (12.9) | 6 (19.4) | 3 (7.7) | ||||
| eGFR (mL/min/1.73 m2), n (%) | 0.042 | ||||||
| Normal (≥60) | 41 (58.6) | 14 (45.2) | 27 (69.2) | ||||
| Low (<60) | 29 (41.4) | 17 (54.8) | 12 (30.8) | ||||
| Hypoalbuminemia (<3.4 g/dL) | 46 (65.7) | 24 (80.0) | 22 (56.4) | 0.045 | 4.900 | 1.239–19.380 | 0.024 |
| Hemodialysis, n (%) | 10 (14.3) | 5 (16.1) | 5 (12.8) | 0.741 | |||
| ECMO, n (%) | 8 (11.4) | 4 (12.9) | 4 (10.3) | 1.0 | |||
| Hemofiltration, n (%) | 2 (2.9) | 2 (6.5) | 0 | 0.193 | |||
| Type of treatment VGCV/GCV, n (%) | |||||||
| Prophylaxis | 14/70 (20) | 0 | 14 (35.9) | 0.001 | |||
| Targeted | 56/70 (80) | 31 (100) | 25 (64.1) | ||||
| Asymptomatic reactivation | 30/56 (53.6) | 18 (58.1) | 12 (30.8) | 0.029 | |||
| CMV syndrome | 11/56 (19.6) | 6 (19.4) | 5 (12.8) | 0.520 | |||
| CMV end-organ disease | 15/56 (26.8) | 7 (22.6) | 8 (20.5) | 1.0 | |||
| Main indications for valganciclovir/ganciclovir CMV end-organ disease, n (%) | |||||||
| Pneumonia | 5/15 (33.3) | 3 (9.7) | 2 (5.1) | 0.649 | |||
| Retinitis | 1/15 (6.7) | 0 | 1 (2.6) | 1.0 | |||
| Hepatitis | 0 | 0 | 0 | NA | |||
| CNS disease | 2/15 (13.3) | 0 | 2 (5.1) | 0.499 | |||
| Nephritis | 0 | 0 | 0 | NA | |||
| Gastrointestinal disease | 6/15 (40) | 3 (9.7) | 3 (7.7) | 1.0 | |||
| Myocarditis | 0 | 0 | 0 | NA | |||
| Cystitis | 0 | 0 | 0 | NA | |||
| Cholangitis | 1/15 (6.7) | 1 (3.2) | 0 | 0.443 | |||
| Pancreatitis | 0 | 0 | 0 | NA | |||
| Basal CMV viral load, median (IQR) ** | 1361 (496.2–9322.5) | 3995 (558–10034) | 742 (404.5–6797) | 0.132 | |||
| Time (days) to negative viremia Median (IQR) | 12.5 (4–21) | 17 (9–23.5) | 7.5 (3–13) | 0.005 | |||
| ICU, days, median (IQR) | 31.0 (15.0–63.0) | 29 (20–50) | 63 (12–133.5) | 0.574 | |||
| Hospital stay, days, median (IQR) | 48.5 (23.0–92.5) | 51 (27–106) | 44 (15–89) | ||||
| Valganciclovir/ganciclovir use | |||||||
| Duration of prophylaxis/treatment (days), median (IQR) | 14.5 (8–22) | 17 (9–22) | 14 (8–21) | 0.663 | |||
| Dose adequacy, n (%) | |||||||
| Adequate dose | 47/70 (67.1) | 16 (51.6) | 31 (79.5) | 0.021 | 4.673 | 1.227–17.798 | 0.024 |
| Non-adequate (infradoses) | 7/70 (10.0) | 5 (16.1) | 2 (5.1) | 0.228 | |||
| Non-adequate (supradoses) | 16/70 (22.9) | 10 (32.3) | 6 (15.4) | 0.151 | |||
| Ganciclovir serum level | |||||||
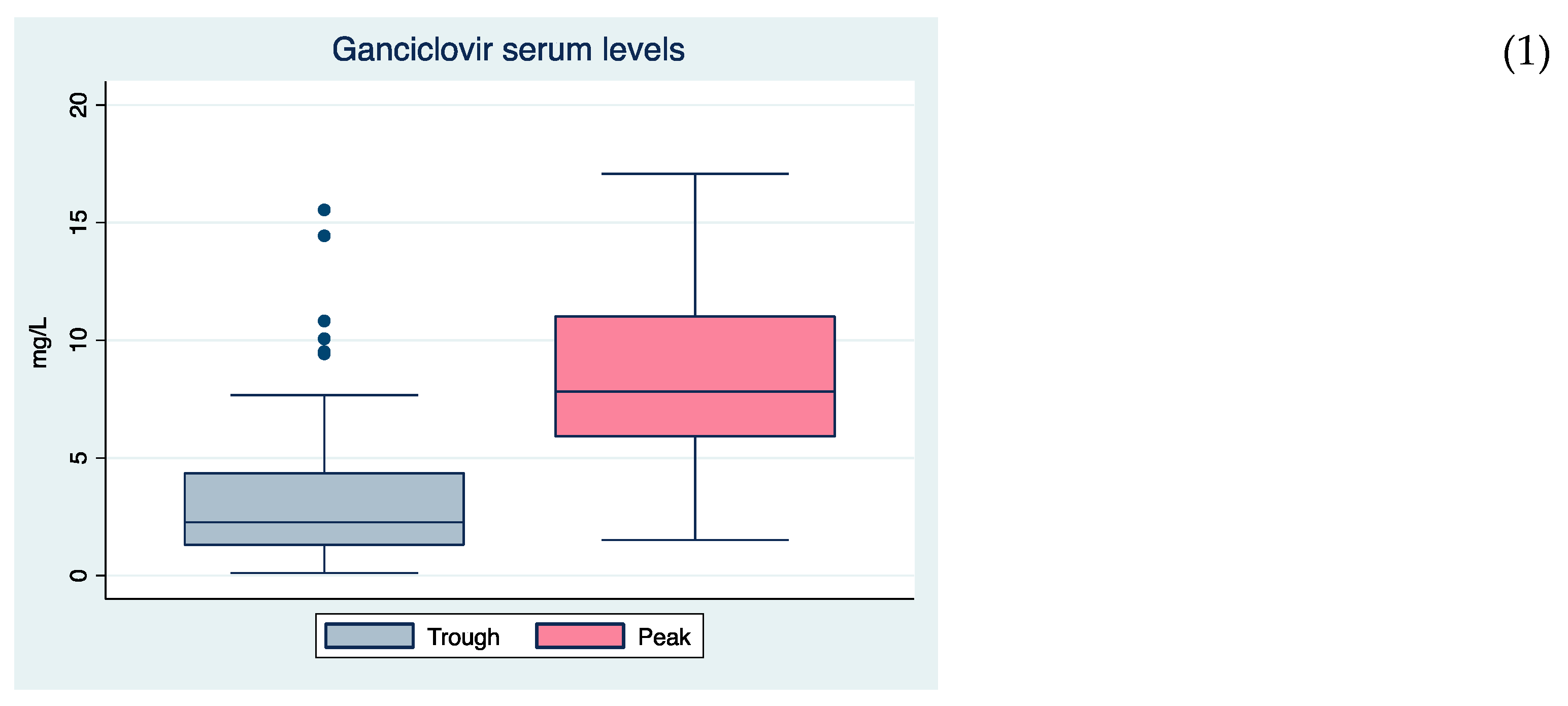
| Cmin (mg/L), median (IQR) | 2.3 (1.3–4.4) | 2.3 (1.4–4.5) | 2.2 (1.2–3.7) | 0.493 | |||
| Cmax (mg/L), median (IQR) | 7.8 (5.8–11.1) | 7.6 (6.2–9.5) | 8.1 (5.4–11.2) | 0.780 | |||
| Cmax <8.37 mg/L or >11.86 mg/L, n (%) | 12 (17.1) | 1 (3.2) | 11 (28.2) | 0.009 | 9.350 | 1.016–86.006 | 0.048 |
| Concomitant medications, n (%) | |||||||
| Probenecid | 1 (1.4) | 0 | 1 (2.6) | 1.0 | |||
| Mycophenolate mofetil | 26 (37.1) | 12 (38.7) | 14 (35.9) | 1.0 | |||
| Zidovudine | 0 | 0 | 0 | NA | |||
| Stavudine | 0 | 0 | 0 | NA | |||
| Didanosine | 0 | 0 | 0 | NA | |||
| Imipenem | 0 | 0 | 0 | NA | |||
| Amphotericin B | 1 (1.4) | 1 (3.2) | 0 | 0.443 | |||
| Trimethoprim/sulfamethoxazole | 33 (47.1) | 14 (45.2) | 19 (48.7) | 0.813 | |||
| Hydroxyurea | 0 | 0 | 0 | NA | |||
| Pentamidine | 0 | 0 | 0 | NA | |||
| Flucytosine | 0 | 0 | 0 | NA | |||
| Vincristine | 1 (1.4) | 1 (3.2) | 0 | 0.443 | |||
| Vinblastine | 1 (1.4) | 1 (3.2) | 0 | 0.443 | |||
| Doxorubicin | 0 | 0 | 0 | NA | |||
| Dapsone | 0 | 0 | 0 | NA | |||
| Tenofovir disoproxil | 4 (5.7) | 2 (6.5) | 2 (5.1) | 1.0 | |||
| Foscarnet | 0 | 0 | 0 | NA | |||
| Cidofovir | 0 | 0 | 0 | NA | |||
| Tacrolimus | 27 (38.6) | 11 (35.5) | 16 (41) | 0.805 | |||
| Cyclosporine | 5 (7.1) | 1 (3.2) | 4 (10.3) | 0.374 | |||
| Everolimus | 3 (4.3) | 0 | 3 (7.7) | 0.249 | |||
| Patients, n (Prophylaxis/Treatment) | Ganciclovir Serum Levels | |||||
|---|---|---|---|---|---|---|
| Prophylaxis | Treatment | p Trough | p Peak | |||
| Trough, median (mg/L, IQR) | Peak, median (mg/L, IQR) | Trough, median (mg/L, IQR) | Peak, median (mg/L, IQR) | |||
| Global cohort, n = 70 (14/56) | 1.7 (1.1–3.0) | 7.9 (5.3–11.2) | 2.4 (1.4–4.5) | 7.8 (5.9–11.2) | ||
| Intensive care unit, n = 15 (2/13) | NA * | NA * | 2.7 (1.3–4.9) | 7.9 (6.8–11.1) | 0.336 | 0.267 |
| ECMO/hemofiltration/hemodialysis, n = 15 (5/10) | 1.1 (0.5–2.3) | 10.6 (6.3–12.4) | 3.2 (1.7–7.2) | 7.9 (6.8–14.3) | 0.924 | 0.101 |
| HIV, n = 8 (0/8) | NA | NA | 3.0 (1.5–4.8) | 5.6 (3.0–7.8) | 0.522 | 0.053 |
| Solid organ transplant, n = 26 (9/17) | 1.2 (0.9–5.3) | 7.4 (5.7–12.0) | 2.7 (1.3–5.0) | 8.2 (4.7–12.8) | 0.784 | 0.603 |
| A. Univariate and multivariate analysis of variables associated with Cmin of valganciclovir/ganciclovir (n = 70) | ||||
| GLOBAL | Univariate Analysis | Multivariate Analysis | ||
| Variable | Unstandardized β-Coefficient (95% CI) | p | Unstandardized β-Coefficient (95% CI) | p |
| Age (years) | 0.022 (−0.015 to 0.059) | 0.239 | ||
| Sex | −0.420 (−2.128 to 1.288) | 0.625 | ||
| Weight (kg) | 0.004 (−0.032 to 0.039) | 0.842 | ||
| Body mass index (m2/kg) | −0.006 (−0.122 to 1.110) | 0.922 | ||
| eGFR (MDRD) abnormal (low) | 1.687 (0.225 to 3.150) | 0.024 | 1.730 (0.3 to 3.160) | 0.018 |
| Dose (mg/kg) | −0.012 (−0.092–0.068) | 0.765 | ||
| Hemodialysis | 1.605 (−0.496 to 3.707) | 0.132 | ||
| ECMO | −0.068 (−2.418 to 2.283) | 0.954 | ||
| Hemofiltration | −1.283 (−5.761 to 3.195) | 0.569 | ||
| Beginning in ICU | 1.023 (−0.782 to 2.829) | 0.262 | ||
| Solid organ transplant | 2.978 (2.044–3.911) | 0.239 | ||
| HIV | −0.243 (−2.592–2.107) | 0.837 | ||
| Hypoalbuminemia | 0.287 (−1.316 to 1.889) | 0.722 | ||
| Co-treatment with: | ||||
| Probenecid | −0.351 (−6.652 to 5.950) | 0.912 | ||
| Mycophenolate mofetil | 1.185 (−0.336 to 2.705) | 0.125 | ||
| Amphotericin B | −2.137 (−0.417 to 4.144) | 0.500 | ||
| Trimethoprim/sulfamethoxazole | 0.773 (−0.714 to 2.259) | 0.303 | ||
| Tenofovir disoproxil | −0.327 (−3.548 to 2.893) | 0.840 | ||
| Tacrolimus | 0.949 (−0.570 to 2.468) | 0.217 | ||
| Cyclosporine | −0.847 (−3.743 to 2.050) | 0.562 | ||
| Everolimus | −1.553 (−5.226 to 2.120) | 0.402 | ||
| Charlson | −0.024 (−0.348 to 0.301) | 0.885 | ||
| McCabe | −0.475 (−1.522 to 0.572) | 0.368 | ||
| B. Univariate and multivariate analysis of variables associated with Cmax of valganciclovir/ganciclovir (n = 70) | ||||
| GLOBAL | Univariate Analysis | Multivariate Analysis | ||
| Variable | Unstandardized β-Coefficient (95% CI) | p | Unstandardized β-Coefficient (95% CI) | p |
| Age (years) | 0.002 (−0.043 to 0.048) | 0.923 | ||
| Sex | 1.024 (−1.075 to 3.124) | 0.334 | ||
| Weight (kg) | −0.020 (−0.063 to 0.023) | 0.364 | ||
| Body mass index (m2/kg) | −0.031 (−0.172 to 0.109) | 0.659 | ||
| eGFR (MDRD) abnormal (low) | 1.898 (0.111 to 3.685) | 0.038 | ||
| Dose (mg/kg) | −0.037 (−0.137–0.063) | 0.464 | ||
| Hemodialysis | 3.173 (0.703 to 5.643) | 0.013 | 3.173 (0.703 to 5.643) | 0.013 |
| ECMO | 1.512 (−1.310 to 4.334) | 0.289 | ||
| Hemofiltration | 2.037 (−3.372 to 7.445) | 0.455 | ||
| Beginning in ICU | 0.995 (−1.201 to 3.190) | 0.369 | ||
| Solid organ transplant | 0.648 (−1.225-2.522) | 0.492 | ||
| HIV | −2.719 (−5.664-0.225) | 0.070 | ||
| Hypoalbuminemia | 0.253 (−1.655 to 2.162) | 0.792 | ||
| Co-treatment with: | ||||
| Probenecid | 1.256 (−6.362 to 8.874) | 0.743 | ||
| Mycophenolate mofetil | 0.324 (−1.554 to 2.203) | 0.732 | ||
| Amphotericin B | −0.327 (−7.951 to 7.297) | 0.932 | ||
| Trimethoprim/sulfamethoxazole | −0.765 (−2.580 to 1.050) | 0.403 | ||
| Tenofovir disoproxil | −3.989 (−8.350 to 0.372) | 0.072 | ||
| Tacrolimus | 0.923 (−0.931 to 2.776) | 0.324 | ||
| Cyclosporine | −0.917 (−4.424 to 2.591) | 0.604 | ||
| Everolimus | 1.277 (−3.181 to 5.734) | 0.569 | ||
| Charlson | −0.115 (−0.514 to 0.284) | 0.567 | ||
| McCabe | −0.978 (−2.235 to 0.280) | 0.126 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galar, A.; Valerio, M.; Catalán, P.; García-González, X.; Burillo, A.; Fernández-Cruz, A.; Zataráin, E.; Sousa-Casasnovas, I.; Anaya, F.; Rodríguez-Ferrero, M.L.; et al. Valganciclovir—Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship. Antibiotics 2021, 10, 77. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010077
Galar A, Valerio M, Catalán P, García-González X, Burillo A, Fernández-Cruz A, Zataráin E, Sousa-Casasnovas I, Anaya F, Rodríguez-Ferrero ML, et al. Valganciclovir—Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship. Antibiotics. 2021; 10(1):77. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010077
Chicago/Turabian StyleGalar, Alicia, Maricela Valerio, Pilar Catalán, Xandra García-González, Almudena Burillo, Ana Fernández-Cruz, Eduardo Zataráin, Iago Sousa-Casasnovas, Fernando Anaya, María Luisa Rodríguez-Ferrero, and et al. 2021. "Valganciclovir—Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship" Antibiotics 10, no. 1: 77. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010077






