Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Patients and Ceftriaxone Concentrations
2.2. Protein Binding Model
2.3. Agreement between Measured CEFu and Predicted CEFu
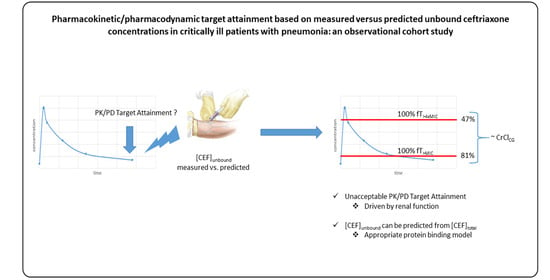
2.4. Ceftriaxone PK/PD Target Attainment
3. Discussion
4. Materials and Methods
4.1. Setting, Study Design and Population
4.2. Study Protocol
4.3. Bioanalysis Method
Unbound Fraction
4.4. Protein Binding Model
4.5. Agreement between Measured CEFu and Predicted CEFu
4.6. PK/PD Target Attainment
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Finelli, L. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M., Jr.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. 2), S27–S72. [Google Scholar] [CrossRef]
- Kaysin, A.; Viera, A.J. Community-Acquired Pneumonia in Adults: Diagnosis and Management. Am. Fam Physician 2016, 94, 698–706. [Google Scholar]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnacho-Montero, J.; Barrero-Garcia, I.; Gomez-Prieto, M.G.; Martin-Loeches, I. Severe community-acquired pneumonia: Current management and future therapeutic alternatives. Expert Rev. Infect. 2018, 16, 667–677. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garot, D.; Respaud, R.; Lanotte, P.; Simon, N.; Mercier, E.; Ehrmann, S.; Perrotin, D.; Dequin, P.F.; Le Guellec, C. Population pharmacokinetics of ceftriaxone in critically ill septic patients: A reappraisal. Br. J. Clin. Pharm. 2011, 72, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Leegwater, E.; Kraaijenbrink, B.V.C.; Moes, D.; Purmer, I.M.; Wilms, E.B. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 1554–1558. [Google Scholar] [CrossRef]
- Ollivier, J.; Carrie, C.; d’Houdain, N.; Djabarouti, S.; Petit, L.; Xuereb, F.; Legeron, R.; Biais, M.; Breilh, D. Are Standard Dosing Regimens of Ceftriaxone Adapted for Critically Ill Patients with Augmented Creatinine Clearance? Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joynt, G.M.; Lipman, J.; Gomersall, C.D.; Young, R.J.; Wong, E.L.; Gin, T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 2001, 47, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.E.; Huttner, B.; Huttner, A. Therapeutic Drug Monitoring of Beta-Lactams and Other Antibiotics in the Intensive Care Unit: Which Agents, Which Patients and Which Infections? Drugs 2018, 78, 439–451. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoeckel, K.; McNamara, P.J.; Brandt, R.; Plozza-Nottebrock, H.; Ziegler, W.H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin. Pharm. 1981, 29, 650–657. [Google Scholar] [CrossRef]
- Schleibinger, M.; Steinbach, C.L.; Topper, C.; Kratzer, A.; Liebchen, U.; Kees, F.; Salzberger, B.; Kees, M.G. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br. J. Clin. Pharm. 2015, 80, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin Pharm. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Bos, J.C.; Prins, J.M.; Misticio, M.C.; Nunguiane, G.; Lang, C.N.; Beirao, J.C.; Mathot, R.A.A.; van Hest, R.M. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: A population pharmacokinetic modelling study. J. Antimicrob. Chemother. 2018, 73, 1620–1629. [Google Scholar] [CrossRef] [Green Version]
- Zeitlinger, M.A.; Derendorf, H.; Mouton, J.W.; Cars, O.; Craig, W.A.; Andes, D.; Theuretzbacher, U. Protein binding: Do we ever learn? Antimicrob. Agents Chemother. 2011, 55, 3067–3074. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xue, J.; Shao, J.; Jia, L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 2012, 17, 475–485. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of beta-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gijsen, M.; Wilmer, A.; Meyfroidt, G.; Wauters, J.; Spriet, I. Can augmented renal clearance be detected using estimators of glomerular filtration rate? Crit. Care 2020, 24, 359. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Curran, R.A.; Denny, K.J.; Sime, F.B.; Stanford, C.L.; McWhinney, B.; Ungerer, J.; Roberts, J.A.; Lipman, J. Ceftriaxone dosing in patients admitted from the emergency department with sepsis. Eur. J. Clin. Pharmacol. 2021, 77, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Stewart, P.; Goud, R.; Gourley, S.; Hewagama, S.; Krishnaswamy, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Total and unbound ceftriaxone pharmacokinetics in critically ill Australian Indigenous patients with severe sepsis. Int. J. Antimicrob. Agents 2016, 48, 748–752. [Google Scholar] [CrossRef]
- Fink, S.; Karp, W.; Robertson, A. Ceftriaxone effect on bilirubin-albumin binding. Pediatrics 1987, 80, 873–875. [Google Scholar]
- Gulian, J.M.; Dalmasso, C.; Pontier, F.; Gonard, V. Displacement effect of ceftriaxone on bilirubin bound to human serum albumin. Chemotherapy 1986, 32, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Mimoz, O.; Soreda, S.; Padoin, C.; Tod, M.; Petitjean, O.; Benhamou, D. Ceftriaxone pharmacokinetics during iatrogenic hydroxyethyl starch-induced hypoalbuminemia: A model to explore the effects of decreased protein binding capacity on highly bound drugs. Anesthesiology 2000, 93, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chow, M.S.; Maderazo, E.G. Characteristics of ceftriaxone binding to immunoglobulin G and potential clinical significance. Antimicrob Agents Chemother 1991, 35, 2232–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page-Sharp, M.; Nunn, T.; Salman, S.; Moore, B.R.; Batty, K.T.; Davis, T.M.; Manning, L. Validation and Application of a Dried Blood Spot Ceftriaxone Assay. Antimicrob. Agents Chemother. 2016, 60, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Ongas, M.; Standing, J.; Ogutu, B.; Waichungo, J.; Berkley, J.A.; Kipper, K. Liquid chromatography-tandem mass spectrometry for the simultaneous quantitation of ceftriaxone, metronidazole and hydroxymetronidazole in plasma from seriously ill, severely malnourished children. Wellcome Open Res. 2017, 2, 43. [Google Scholar] [CrossRef] [Green Version]
- Wongchang, T.; Winterberg, M.; Tarning, J.; Sriboonvorakul, N.; Muangnoicharoen, S.; Blessborn, D. Determination of ceftriaxone in human plasma using liquid chromatography–tandem mass spectrometry. Wellcome Open Res. 2019, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Rehngren, M. High-throughput screening of protein binding by equilibrium dialysis combined with liquid chromatography and mass spectrometry. J. Chromatogr. 2006, 1102, 125–134. [Google Scholar] [CrossRef]
- Reynolds, J.E. Martindale: The Extra Pharmacopoeia, 13th ed.; The Pharmaceutical Press: London, UK, 1993. [Google Scholar]
- Sabbioni, G.; Turesky, R.J. Biomonitoring Human Albumin Adducts: The Past, the Present, and the Future. Chem. Res. Toxicol. 2017, 30, 332–366. [Google Scholar] [CrossRef]
- Jager, N.G.L.; van Hest, R.M.; Xie, J.; Wong, G.; Ulldemolins, M.; Bruggemann, R.J.M.; Lipman, J.; Roberts, J.A. Optimization of flucloxacillin dosing regimens in critically ill patients using population pharmacokinetic modelling of total and unbound concentrations. J. Antimicrob. Chemother. 2020, 75, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.J.; Bullock, F.J.; Schoenemann, P.T. Numerical solution of nonlinear pharmacokinetic equations: Effects of plasma protein binding on drug distribution and elimination. J. Pharm. Sci. 1971, 60, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Trueb, V.; Stoeckel, K. Protein binding of ceftriaxone in extravascular fluids. J. Pharm. Sci. 1988, 77, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Applying the right statistics: Analyses of measurement studies. Ultrasound. Obs. Gynecol. 2003, 22, 85–93. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Dulhunty, J.M.; Bellomo, R.; Lipman, J.; Roberts, J.A. Continuous beta-lactam infusion in critically ill patients: The clinical evidence. Ann. Intensive Care 2012, 2, 37. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Overall | On early Sampling Day | On late Sampling Day |
|---|---|---|---|
| Number of patients, n (%) a | 31 (100) | 25 (81) | 11 (35) |
| Demographics | |||
| Sex, male, n (%) | 19 (61) | ||
| Age, median (IQR), years | 72 (55–81) | ||
| Body weight, median (IQR), kg | 64 (60–77) | ||
| Clinical scores | |||
| Sequential Organ Failure Assessment score, median (IQR), n | 6 (4–9) | 8 (6–10), 15 | 5 (3–6), 11 |
| Acute physiology and chronic health evaluation II score, median (IQR) | 18 (16–26) | ||
| Biochemical parameters | |||
| Serum creatinine, median (IQR), mg/dL, n | 0.86 (0.71–1.04) | 0.91 (0.71–1.15), 25 | 0.8 (0.71–0.87), 11 |
| Cockcroft–Gault equation, median (IQR), mL/min, n | 73 (54–102) | 71 (49–90), 25 | 87 (58–109), 11 |
| Serum albumin, median (IQR), g/L, n | 29.5 (26.7–31.7) | 29.5 (26.8–31.6), 25 | 29.6 (26.2–31.5), 11 |
| Total bilirubin, median (IQR), mg/dL, n | 0.8 (0.25–0.6) | 0.38 (0.26–0.57), 25 | 0.28 (0.24–0.68), 11 |
| Sampling | |||
| Unbound ceftriaxone pre-dose concentration ≥1 mg/L, n (%) | 26/32 (81) | 17/21 (81) | 9/11 (81.8) |
| Unbound ceftriaxone pre-dose concentration ≥4 mg/L, n (%) | 15/32 (47) | 10/21 (47.6) | 5/11 (45.5) |
| CEFu Predicted mg/L, Median (IQR) | fu % Predicted, Median (IQR) | Bias mg/L, Median (IQR) | Relative Bias, % of CEFu, Median (IQR) | Relative RMSE, % of CEFu | p-Value b | |
|---|---|---|---|---|---|---|
| Fixed average protein binding | 8.3 (3.4–13.9) | 10.5 a | 5.6 (0.6–12) | 38.2 (19.2–50.6) | 86.1 | <0.0001 |
| Predicted saturable concentration-dependent protein binding, Equation (4) | 5 (1.8–10) | 6.3 (5.5–7.6) | 8.4 (2.2–15.7) | 59.2 (50.9–68) | 89 | <0.0001 |
| Predicted protein binding, present study, Equation (2) | 15.5 (4.2–30.4) | 17.9 (12.3–22.6) | 0.05 (−0.8–1.1) | 0.4 (−5.8–12.6) | 14.2 | 0.627 |
| Predicted protein binding, Bos | 21.8 (3.5–62.1) | 28.2 (11.7–45.3) | −6.2 (−35.9–0.1) | −35.9 (−143.7–4.8) | 195.3 | <0.0001 |
| Predicted protein binding, Leegwater | 11.5 (3.2–33.4) | 14.7 (10–25.2) | 0.3 (−10.1–1.5) | 10.2 (−49.5–29.3) | 109.2 | 0.350 |
| 100% fT>MIC, n (%) | p-Value a | 100% fT>4xMIC, n (%) | p-Value a | |
|---|---|---|---|---|
| Fixed average protein binding | 26 (81.2) | 1.000 | 11 (34.4) | 0.044 |
| Predicted saturable concentration-dependent protein binding, Equation (4) | 23 (71.2) | 0.083 | 5 (15.6) | 0.0007 |
| Predicted protein binding, present study, Equation (2) | 24 (75) | 0.572 | 13 (40.6) | 1.000 |
| Predicted protein binding, Bos | 25 (78.1) | 0.325 | 13 (40.6) | 0.325 |
| Predicted protein binding, Leegwater | 25 (78.1) | 0.325 | 11 (34.4) | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gijsen, M.; Dreesen, E.; Van Daele, R.; Annaert, P.; Debaveye, Y.; Wauters, J.; Spriet, I. Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study. Antibiotics 2021, 10, 557. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050557
Gijsen M, Dreesen E, Van Daele R, Annaert P, Debaveye Y, Wauters J, Spriet I. Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study. Antibiotics. 2021; 10(5):557. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050557
Chicago/Turabian StyleGijsen, Matthias, Erwin Dreesen, Ruth Van Daele, Pieter Annaert, Yves Debaveye, Joost Wauters, and Isabel Spriet. 2021. "Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study" Antibiotics 10, no. 5: 557. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050557