Current Antimicrobial Stewardship Practice and Education in Russian Hospitals: Results of a Multicenter Survey
Abstract
:1. Introduction
- the current level of postgraduate educational and training activity amongst healthcare practitioners involved in AMS in Russia;
- the availability of human resources to support AMS;
- the organization of education and training within Russian hospitals;
- the demand for AMS education, including the organization, content and preferred methods of delivery.
2. Methods and Material
2.1. Study Design
2.2. Piloting
2.3. Questionnaire Layout
- respondent’s main characteristics
- –
- geographical work location
- –
- type of organization
- –
- medical specialty (clinical pharmacology/microbiology/pharmacy/public health/epidemiology/surgery/therapy/etc.)
- –
- profession (doctor/pharmacist/nurse/other)
- –
- role in antimicrobial stewardship (prescription/administration/education/etc.)
- personal education and training in AMS (undergraduate and postgraduate)
- institution/organization main characteristics
- –
- description of microbiological service
- –
- AMS team availability
- organization of education and training in AMS in the institution/organization
- –
- characteristics of educational process
- –
- organizational frameworks
- –
- covered topics at induction/during employment
- –
- methods of education
- demands in AMS (preferred topics, learning methods and needs)
2.4. Data Collection and Analysis
2.5. Ethics
3. Results
3.1. Geographical Distribution of Participating Centers
3.2. Main Characteristics of Survey Respondents
3.3. Personal Education and Training in Antimicrobial Stewardship
3.4. Role of Microbiologists and AMS Program Implementation in Institution/Organization
3.5. Organization of Antimicrobial Stewardship Teaching at the Institution Level
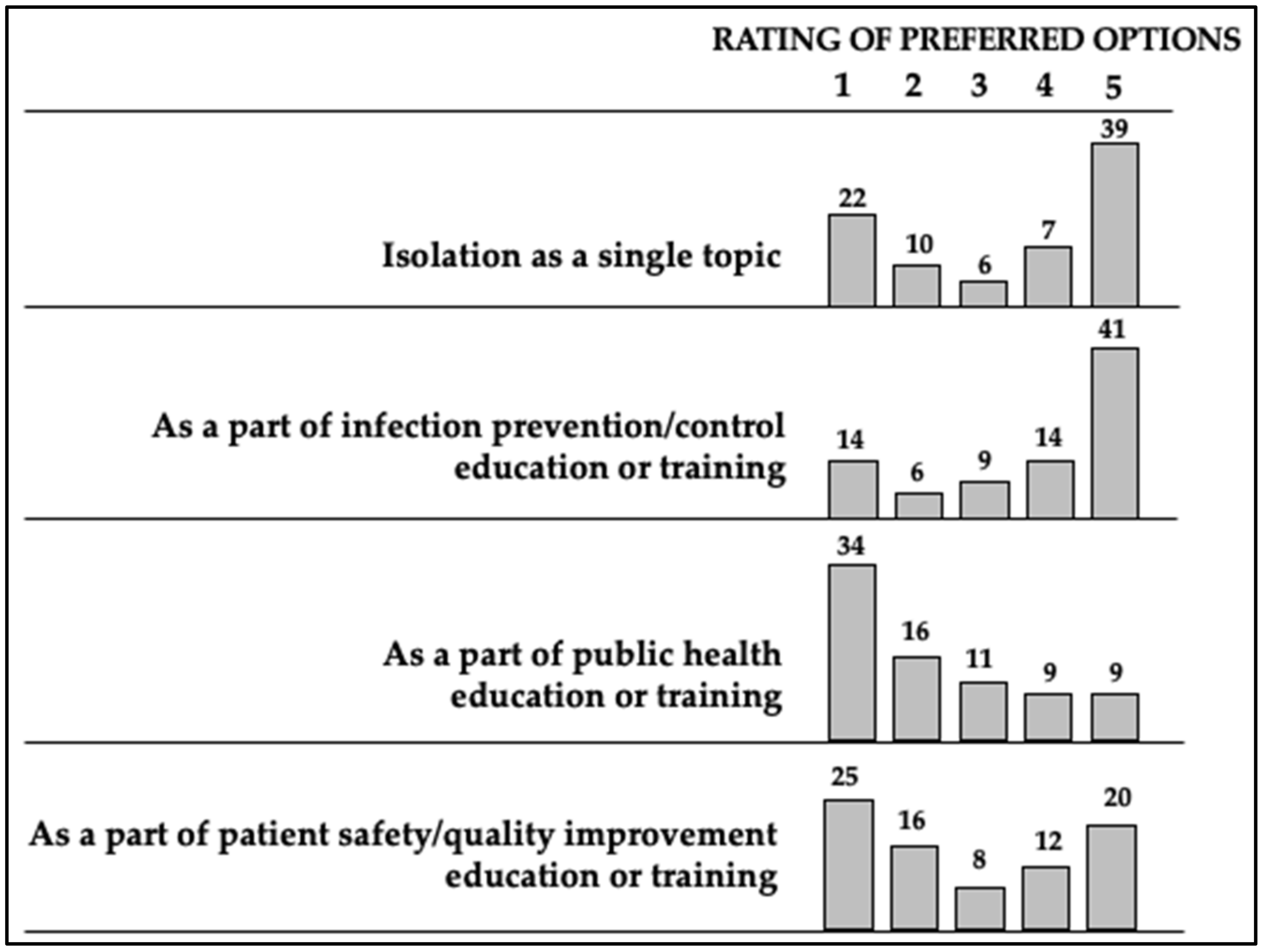
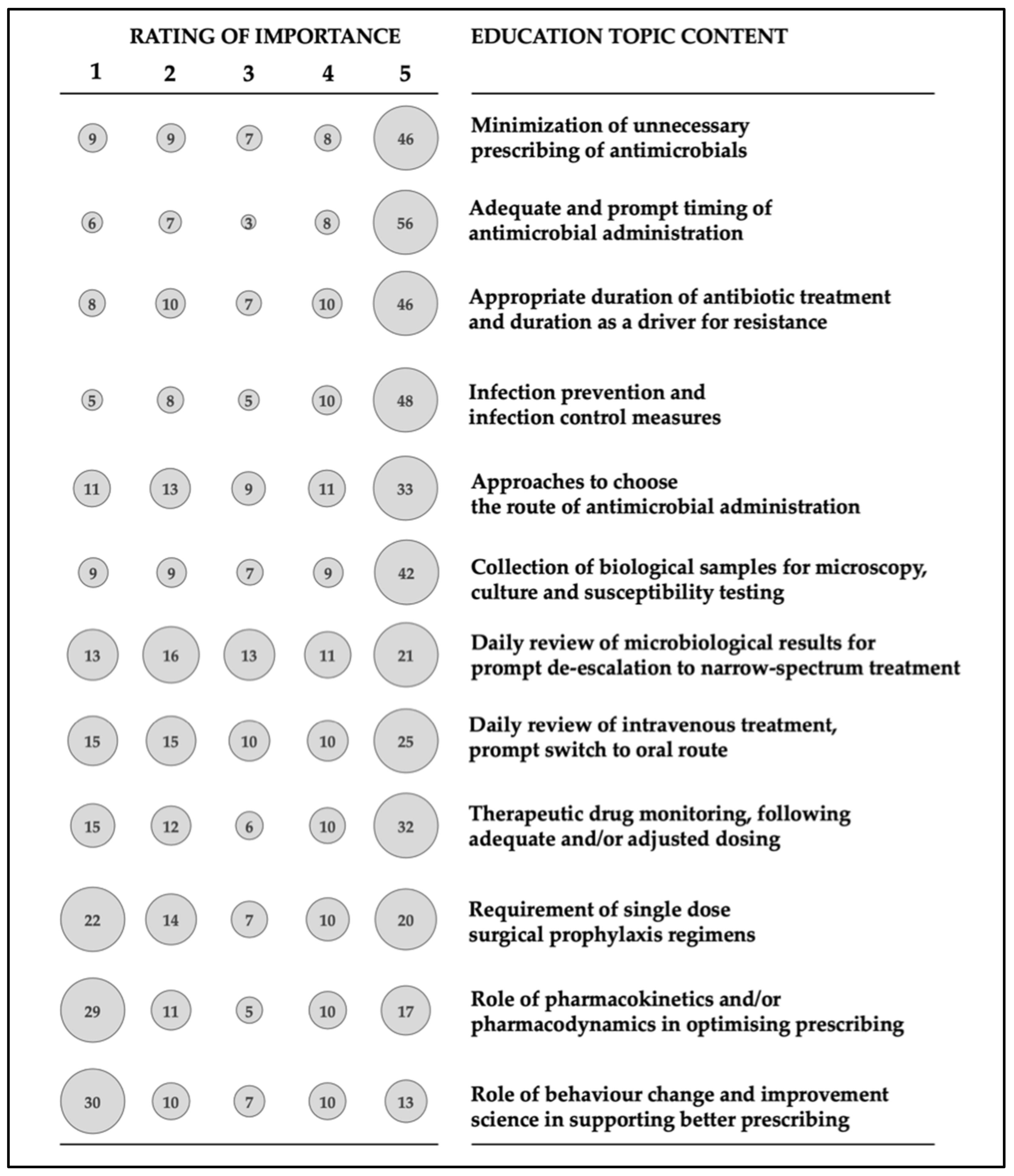
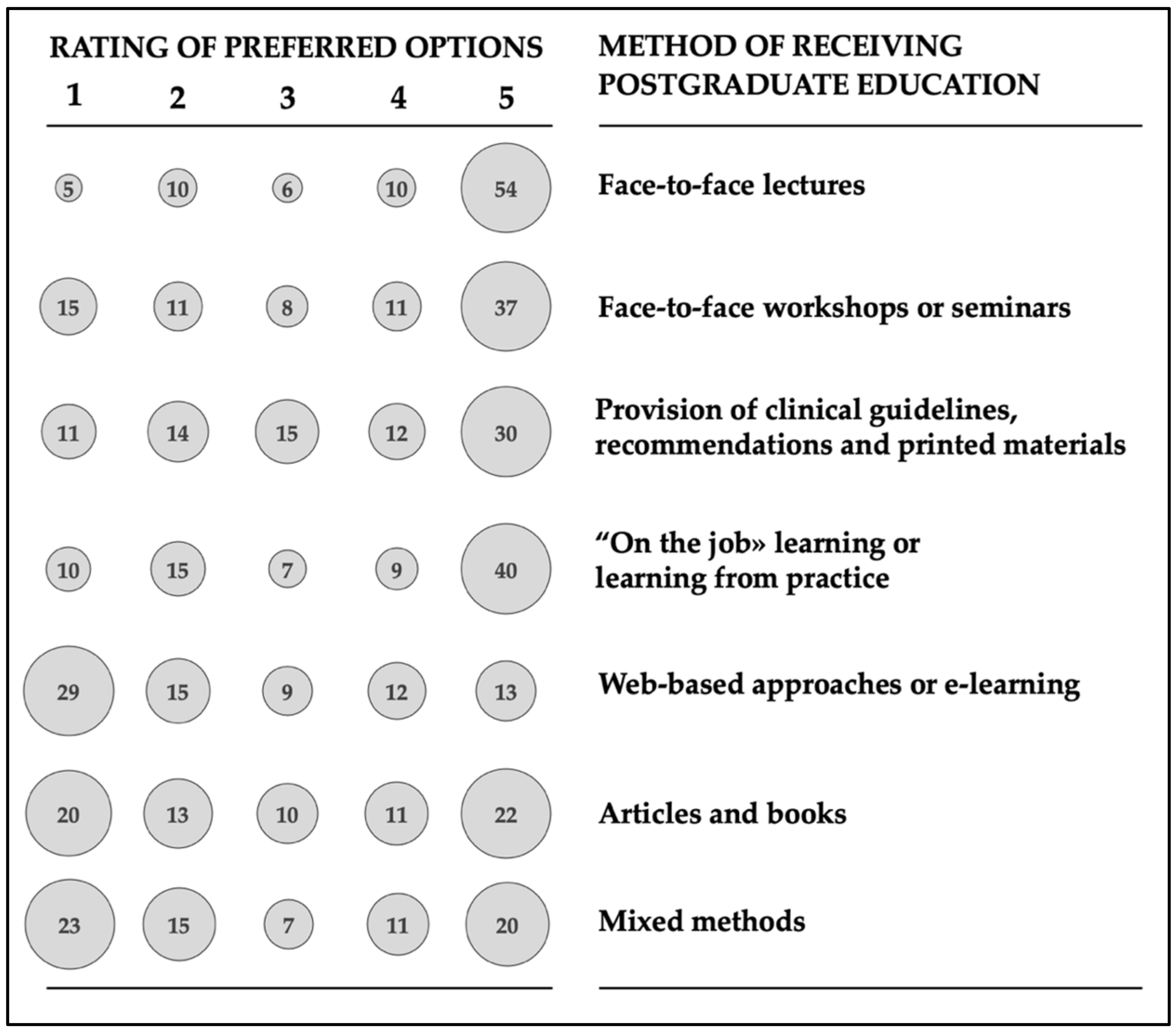
3.6. Personal Preferences for Antimicrobial Stewardship Education/Training
- Minimization of unnecessary prescribing of antimicrobials
- Adequate and prompt timing of antimicrobial administration
- Appropriate duration of antibiotic treatment and duration as a driver for resistance
- Infection prevention and infection control measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action. 2012. Available online: https://apps.who.int/iris/handle/10665/44812 (accessed on 29 May 2020).
- Kamarudin, G.; Penm, J.; Chaar, B.; Moles, R. Educational interventions to improve prescribing competency: A systematic review. BMJ Open 2013, 3, e003291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, L.M.; Cosgrove, S.E.; Pottinger, P.S.; Pereyra, M.; Sinkowitz-Cochran, R.; Srinivasan, A.; Webb, D.J.; Hooton, T.M. Medical students’ perceptions and knowledge about antimicrobial stewardship: How are we educating our future prescribers? Clin. Infect. Dis. 2013, 57, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Olans, R.N.; Olans, R.D.; DeMaria, A., Jr. The critical role of the staff nurse in antimicrobial stewardship—Unrecognized, but already there. Clin. Infect. Dis. 2016, 62, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duin, D.; Barlow, G.; Nathwani, D. The impact of the COVID-19 pandemic on antimicrobial resistance: A debate. J. Antimicrob. Chemother. Antimicrob. Resist. 2020, 2, dlaa053. [Google Scholar] [CrossRef]
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. J. Antimicrob. Chemother. Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef]
- AntiMicrobial Resistance Map. Available online: https://amrmap.net/ (accessed on 10 July 2020).
- Gusarov, V.G.; Nesterova, E.E.; Oprishenko, I.V.; Petrova, N.V.; Zamyatin, M.N. Clinical and pharmacoeconomic results of the use of the protocol empiric antimicrobial therapy in a multidisciplinary hospital. Bull. Pirogov Nat. Med. Surg. Cent. 2015, 10, 100–104. (In Russian) [Google Scholar]
- Rudnov, V.A.; Kolotova, G.B.; Bagin, V.A.; Nevskaya, N.N.; Belsky, D.V.; Ivanova, N.A.; Gayfutdinov, E.A. The role of antimicrobial therapy stewardship in intensive care service. Clin. Microbiol. Antimicrob. Chemother. 2018, 20, 132–140. [Google Scholar] [CrossRef]
- Bodyaeva, E.V.; Rachina, S.A.; Otvagin, I.V.; Gudkov, I.V. Efficacy of Intervention Campaign in Outpatients with Acute Tonsillopharyngitis in Smolensk. Clin. Microbiol. Antimicrob. Chemother. 2011, 13, 46–55. [Google Scholar]
- Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 29 May 2020).
- National Strategy for Combating Antimicrobial Resistance in Russia until 2030. Available online: http://static.government.ru/media/files/onJ3GY3ObDGqLDvrED7AhpLF3ywRRFpp.pdf (accessed on 29 May 2020).
- Kuzmenkov, A.Y.; Trushin, I.V.; Vinogradova, A.G.; Avramenko, A.A.; Sukhorukova, M.V.; Malhotra-Kumar, S.; Dekhnich, A.V.; Edelstein, M.V.; Kozlov, R.S. AMRmap: An Interactive Web Platform for Analysis of Antimicrobial Resistance Surveillance Data in Russia. Front. Microbiol. 2021, 12, 620002. [Google Scholar] [CrossRef]
- U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria (National Action Plan). Available online: https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html (accessed on 29 May 2020).
- Antimicrobial Resistance: UK Launches 5-Year Action Plan and 20-Year Vision. Available online: https://www.gov.uk/government/news/antimicrobial-resistance-uk-launches-5-year-action-plan-and-20-year-vision (accessed on 29 May 2020).
- Charani, E.; Smith, I.; Skodvin, B.; Perozziello, A.; Lescure, F.-X.; Lucet, J.-C.; Birgand, G.; Poda, A.; Ahmad, R.; Singh, S.; et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—A qualitative study. PLoS ONE 2019, 14, e0209847. [Google Scholar] [CrossRef]
- Petrov, V.I.; Kagramanyan, I.N.; Khokhlov, A.L.; Frolov, M.U.; Lileeva, E.G. Development of Clinical Pharmacology in the Russian Federation. Clin. Ther. 2016, 38, 1218–1226. [Google Scholar] [CrossRef]
- Yakovlev, S.V.; Briko, N.I.; Sidorenko, S.V.; Protsenko, D.N. SCAT Program (Antimicrobial Therapy Control Strategy) in the Provision of Inpatient Medical Care: Russian Clinical Guidelines; Publishing House “Pero”: Moscow, Russia, 2018; 156p. (In Russian) [Google Scholar]
- Gyssens, I.C. Role of Education in Antimicrobial Stewardship. Med. Clin. N. Am. 2018, 102, 855–871. [Google Scholar] [CrossRef]
- Cunha, C.B. Antimicrobial Stewardship Programs: Principles and Practice. Med. Clin. N. Am. 2018, 102, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Charani, E.; Wattal, C.; Arora, A.; Jenkins, A.; Nathwani, D. The State of Education and Training for Antimicrobial Stewardship Programs in Indian Hospitals—A Qualitative and Quantitative Assessment. Antibiotics 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Maraolo, A.E.; Ong, D.S.Y.; Cimen, C.; Howard, P.; Kofteridis, D.P.; Schouten, J.; Mutters, N.T.; Pulcini, C. Organization and training at national level of antimicrobial stewardship and infection control activities in Europe: An ESCMID cross-sectional survey. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2061–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratchina, S.A.; Mischenko, V.M.; Bodyaeva, E.V.; Fokin, A.A. Intervention Campaign Efficacy in Outpatients with Acute Tonsillopharyngitis in Smolensk Region. Pharmacoepidemiol. Drug Saf. 2011, 20, 188. [Google Scholar]
- Zamyatin, M.; Gusarov, V.; Petrova, N.; Nesterova, E.; Shilkin, D.; Dementienko, M.; Lashenkova, N. Results of antimicrobial stewardship programme implementation in multidisciplinary hospital. ICU Manag. Pract. 2018, 18, 125–127. [Google Scholar]
- Davey, P.; Brown, E.; Charani, E.; Fenelon, L.; Gould, I.M.; Holmes, A.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, R., Jr.; Kwobah, C.; Muro, F.; Ruwanpathirana, A.; Lyamuya, F.; Bodinayake, C.; Nagahawatte, A.; Piyasiri, B.; Sheng, T.; Bollinger, J.; et al. Barriers to implementing antimicrobial stewardship programs in three low- and middle-income country tertiary care settings: Findings from a multi-site qualitative study. Antimicrob. Resist. Infect. Control 2021, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Rachina, S.; Belkova, Y.; Kozlov, R.; Versporten, A.; Pauwels, I.; Goossens, H.; Bochanova, E.; Domanskaya, O.; Elokhina, E.; Ezhova, L.; et al. Longitudinal Point Prevalence Survey of Antimicrobial Consumption in Russian Hospitals: Results of the Global-PPS Project. Antibiotics 2020, 9, 446. [Google Scholar] [CrossRef]
- Kaae, S.; Ghazaryan, L.; Pagava, K.; Korinteli, I.; Makalkina, L.; Zhetimkarinova, G.; Ikhambayeva, A.; Tentiuc, E.; Ratchina, S.; Zakharenkova, P.; et al. The antibiotic knowledge, attitudes and behaviors of patients, doctors and pharmacists in the WHO Eastern European region—A qualitative, comparative analysis of the culture of antibiotic use in Armenia, Georgia, Kazakhstan, Moldova, Russia and Tajikistan. Res. Soc. Adm. Pharm. 2020, 16, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, N.; Lafferty, N.; Nathwani, D. Educating healthcare professionals in antimicrobial stewardship: Can online-learning solutions help? J. Antimicrob. Chemother. 2015, 70, 3175–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathwani, D.; Guise, T.; Gilchrist, M. E-learning for global antimicrobial stewardship. Lancet Infect. Dis. 2017, 17, 579. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, J.; Barlow, G.; Bradley, S.; Brink, A.; Chandy, S.J.; Nathwani, D. Development and impact of a massive open online course (MOOC) for antimicrobial stewardship. J. Antimicrob. Chemother. 2018, 73, 1091–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massive Open Online Course “Antimicrobial Stewardship: Managing Antimicrobial Resistance”. Available online: https://www.futurelearn.com/courses/antimicrobial-stewardship-russian (accessed on 29 May 2020).
- Massive Open Online Course “Antimicrobial Stewardship for Africa”. Available online: https://www.futurelearn.com/courses/antimicrobial-stewardship-for-africa (accessed on 29 May 2020).
| Federal District | City | n | % |
|---|---|---|---|
| Southern | Krasnodar | 637 | 46.9 |
| Northwestern | Murmansk | 191 | 14.1 |
| Far Eastern | Yakutsk | 239 | 17.6 |
| Siberian | Ulan-Ude | 160 | 11.8 |
| Novokuznetsk | 52 | 3.8 | |
| Ural | Ekaterinburg | 79 | 5.8 |
| Total | - | 1358 | 100 |
| Characteristics | Krasnodar (n = 637) | Murmansk (n = 191) | Yakutsk (n = 239) | Ulan-Ude (n = 160) | Novokuznetsk (n = 52) | Ekaterinburg (n = 79) | Total (n = 1358) |
|---|---|---|---|---|---|---|---|
| Profession | |||||||
| Nurse | 84.1 | 37.7 | 27.2 | 20.6 | - | 77.2 | 52.8 |
| Doctor | 15.5 | 44.5 | 64.9 | 73.8 | 100 | 13.9 | 42.0 |
| Microbiologist | 0.3 | 3.1 | 2.5 | 0.6 | - | - | 1.1 |
| Pharmacist | - | 0.5 | 1.7 | 3.1 | - | 1.3 | 0.8 |
| Other | 3.3 | 14.1 | 3.8 | 1.9 | - | 7.6 | 3.3 |
| Type of organization | |||||||
| Public or governmental hospital | 99.1 | 95.8 | 96.2 | 100 | 84.6 | 100 | 97.7 |
| Primary care center (outpatient department) | - | 3.7 | 2.9 | - | 15.4 | - | 1.6 |
| Perinatal/maternity welfare clinic | 0.9 | - | - | - | - | - | 0.4 |
| Private hospital | - | 0.5 | 0.4 | - | - | - | 0.2 |
| Research institution | - | - | 0.4 | - | - | - | 0.1 |
| Medical specialty | |||||||
| Internal medicine | 37.1 | 27.8 | 25.5 | 33.1 | 17.3 | 15.2 | 31.2 |
| Surgery | 8.3 | 30.9 | 37.7 | 31.9 | 13.5 | 12.7 | 19.9 |
| Intensive care medicine | 24.0 | 0.5 | 13.4 | 16.3 | 32.7 | 39.2 | 19.2 |
| Obstetrics/gynaecology | 23.2 | 0.5 | 0.8 | 0.6 | - | 1.3 | 11.2 |
| Microbiology | 0.8 | 15.2 | 4.6 | 1.3 | - | 6.3 | 3.8 |
| Healthcare administration | - | 2.6 | 4.6 | 3.8 | 1.9 | 3.8 | 1.9 |
| Clinical pharmacology | 0.2 | 2.1 | 0.8 | 1.9 | - | - | 0.7 |
| Pharmacy | - | - | 1.3 | 3.1 | - | 1.3 | 0.7 |
| Epidemiology | - | 1.6 | 0.4 | 0.6 | - | 1.3 | 0.1 |
| Other | 6.4 | 19.4 | 10.9 | 7.5 | 34.6 | 19.2 | 11.0 |
| Part of job in relation to antimicrobials (multiple choice answers) | |||||||
| Prescription | 14.8 | 26.2 | 49.0 | 55.0 | 94.2 | 67.1 | 33.2 |
| Monitoring of the need and appropriateness of antimicrobials during therapy | 7.7 | 16.2 | 24.7 | 23.8 | 38.5 | 29.1 | 16.2 |
| Teaching about infection diagnosis and treatment | 0.3 | 4.2 | 6.7 | 16.3 | 15.4 | 17.7 | 5.5 |
| Development of antimicrobial prescription policy and guidelines | 0.6 | 2.6 | 2.5 | 3.8 | 1.9 | 2.5 | 1.8 |
| Purchase | - | 0.5 | 2.5 | 5.0 | 1.9 | 2.5 | 1.3 |
| None of the above | 85.1 | 56.5 | 38.5 | 28.1 | 1.9 | 21.5 | 59.3 |
| Characteristics | Krasnodar (n = 637) | Murmansk (n = 191) | Yakutsk (n = 239) | Ulan-Ude (n = 160) | Novokuznetsk (n = 52) | Ekaterinburg (n = 79) | Total (n = 1358) |
|---|---|---|---|---|---|---|---|
| Undergraduate education | |||||||
| Yes | 47.9 | 55.5 | 69.5 | 71.9 | 78.9 | 54.4 | 57.1 |
| No | 51.0 | 26.7 | 14.2 | 15.0 | 11.5 | 20.3 | 33.6 |
| Not sure | 1.1 | 2.1 | 11.3 | 6.9 | 9.6 | 17.7 | 5.0 |
| Not relevant | - | 5.8 | 2.9 | 2.5 | - | 3.8 | 1.8 |
| No answer | - | 10.0 | 2.1 | 3.8 | - | 3.8 | 2.4 |
| Postgraduate education | |||||||
| Yes | 26.5 | 33.0 | 47.7 | 61.3 | 82.7 | 43.0 | 38.4 |
| Provided by employing hospital | 19.9 | 20.9 | 35.6 | 50.6 | 63.5 | 24.1 | 28.4 |
| Provided by medical university/college | 16.5 | 13.6 | 36.4 | 31.9 | 32.7 | 21.5 | 22.3 |
| Scientific conference | 5.2 | 11.5 | 15.5 | 28.1 | 42.3 | 17.7 | 12.7 |
| Provided by professional organization/society | 2.4 | 2.6 | 5.0 | 11.9 | 25.0 | 3.8 | 4.9 |
| Provided by another hospital | 1.9 | 1.1 | 3.4 | 11.3 | 5.8 | 8.9 | 3.7 |
| Provided by national/regional governmental agency | 1.3 | 1.6 | 9.2 | 7.5 | 1.9 | 1.3 | 3.5 |
| Provided by pharmaceutical company | 0.6 | 1.6 | 4.6 | 8.1 | 15.4 | 7.6 | 3.3 |
| No | 72.2 | 47.1 | 36.4 | 21.9 | 11.5 | 41.8 | 52.4 |
| Not sure | 1.1 | 5.8 | 13.4 | 3.1 | 1.9 | 11.4 | 4.8 |
| Not relevant | 0.2 | 4.7 | 2.1 | - | - | 2.5 | 1.3 |
| No answer | - | 9.4 | 0.4 | 13.8 | 3.9 | 1.3 | 3.2 |
| Characteristics | Krasnodar (n = 637) | Murmansk (n = 191) | Yakutsk (n = 239) | Ulan-Ude (n = 160) | Novokuznetsk (n = 52) | Ekaterinburg (n = 79) | Total (n = 1358) |
|---|---|---|---|---|---|---|---|
| Clinical component in work of microbiologist (ward rounds, prescribing etc.) | |||||||
| Yes | 64.5 | 18.9 | 40.6 | 35.0 | 30.8 | 21.5 | 46.6 |
| No | 32.0 | 41.2 | 37.2 | 37.5 | 48.1 | 60.8 | 37.2 |
| Not sure | 3.5 | 28.8 | 20.9 | 22.5 | 19.2 | 16.5 | 13.7 |
| No answer | - | 11.0 | 1.3 | 5.0 | 1.9 | 1.3 | 2.5 |
| Antimicrobial stewardship committee/group availability | |||||||
| Yes | 80.4 | 60.2 | 62.8 | 74.4 | 75.0 | 63.3 | 72.5 |
| No | 15.7 | 6.3 | 6.7 | 10.6 | 3.9 | 8.9 | 11.3 |
| Not sure | 3.9 | 28.3 | 29.7 | 9.4 | 21.2 | 25.3 | 14.4 |
| No answer | - | 5.2 | 0.8 | 5.6 | - | 2.5 | 1.7 |
| Initiatives/interventions targeting antimicrobial prescribing availability | |||||||
| Yes | 79.0 | 48.2 | 64.0 | 64.4 | 80.8 | 46.8 | 68.5 |
| Introduced by doctors | 88.7 | 54.5 | 78.7 | 79.4 | 96.2 | 88.6 | 81.3 |
| Introduced by microbiologists | 83.7 | 44.0 | 50.2 | 43.8 | 30.8 | 36.7 | 62.7 |
| Introduced by pharmacists | 82.3 | 45.6 | 47.7 | 50.0 | 30.8 | 27.9 | 62.1 |
| Introduced by epidemiologists | 79.3 | 61.8 | 27.2 | 34.4 | 32.7 | 29.1 | 57.7 |
| Introduced by clinical pharmacologists | - | 8.4 | 20.1 | 11.9 | 15.4 | 3.8 | 6.9 |
| Introduced by nurses | 1.9 | 7.3 | 11.3 | 18.1 | 7.7 | 3.8 | 6.6 |
| No | 16.8 | 7.9 | 7.5 | 6.3 | 3.9 | 19.0 | 12.3 |
| Not sure | 4.2 | 31.9 | 26.8 | 19.4 | 11.5 | 31.7 | 15.8 |
| No answer | - | 12.0 | 1.7 | 10.0 | 3.9 | 2.5 | 3.5 |
| Characteristics | Krasnodar (n = 637) | Murmansk (n = 191) | Yakutsk (n = 239) | Ulan-Ude (n = 160) | Novokuznetsk (n = 52) | Ekaterinburg (n = 79) | Total (n = 1358) |
|---|---|---|---|---|---|---|---|
| Formal strategy or framework for delivering education in antimicrobial stewardship | |||||||
| Yes | 76.0 | 32.5 | 54.0 | 71.3 | 65.4 | 39.2 | 62.9 |
| No | 19.3 | 12.6 | 8.0 | 3.8 | 7.7 | 25.3 | 14.4 |
| Not sure | 4.7 | 46.6 | 37.7 | 16.3 | 26.9 | 34.2 | 20.3 |
| No answer | - | 8.4 | 0.4 | 8.8 | - | 1.3 | 2.4 |
| Institutional education on AMS at induction (within 3 months of starting job) | |||||||
| Yes | 17.6 | 11.5 | 23.4 | 64.4 | 42.3 | 13.9 | 24.0 |
| No | 79.6 | 45.0 | 35.6 | 8.1 | 21.2 | 59.5 | 55.2 |
| Not sure | 2.8 | 33.0 | 39.8 | 13.1 | 30.8 | 22.8 | 17.0 |
| No answer | - | 10.5 | 1.3 | 14.4 | 5.8 | 3.8 | 3.8 |
| Institutional education on AMS during employment | |||||||
| Yes | 58.7 | 35.1 | 54.0 | 78.8 | 88.5 | 41.8 | 57.1 |
| No | 35.8 | 15.7 | 15.9 | 9.4 | - | 30.4 | 24.7 |
| Not sure | 5.5 | 36.1 | 29.7 | 6.9 | 7.7 | 25.3 | 15.5 |
| No answer | - | 13.1 | 0.4 | 5.0 | 3.9 | 2.5 | 2.8 |
| At Induction | During Employment | |
|---|---|---|
| Covered topics | ||
| Minimize unnecessary prescribing of antimicrobials | 18 | 34 |
| Ensure adequate and prompt timing of antimicrobial administration | 23 | 38 |
| Ensure appropriate duration of antibiotic treatment and duration as a driver for resistance | 19 | 34 |
| Adopt necessary infection prevention and control measures | 22 | 49 |
| Intravenous administration only in severely ill and/or those unable to tolerate oral treatment | 13 | 33 |
| Obtain biological samples for microscopy, culture and sensitivity testing | 14 | 38 |
| Review micro results daily, deescalate to narrow-spectrum treatment promptly | 8 | 16 |
| Review intravenous treatment daily, switch to oral route promptly | 8 | 16 |
| Therapeutic drug monitoring, following adequate and/or adjusted dosing | 12 | 20 |
| Require single dose surgical prophylaxis regimens as appropriate | 9 | 18 |
| The role of pharmacokinetics and/or pharmacodynamics in optimizing prescribing | 9 | 14 |
| The role of behavior change and improvement science in supporting better prescribing | 5 | 10 |
| Methods of education and training | ||
| Face-to-face lectures and presentations | 17 | 55 |
| Face-to-face workshops and seminars | 9 | 29 |
| Providing with clinical guidelines, recommendations and printed materials | 17 | 41 |
| “On the job” learning or learning from practice | 17 | 4 |
| Web-based or e-learning | 4 | 8 |
| Mixed methods | 5 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palagin, I.; Rachina, S.; Sukhorukova, M.; Nizhegorodtseva, I.; Portnyagina, U.; Gordeeva, S.; Burasova, E.; Bagin, V.; Domanskaya, O.; Nathwani, D.; et al. Current Antimicrobial Stewardship Practice and Education in Russian Hospitals: Results of a Multicenter Survey. Antibiotics 2021, 10, 892. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10080892
Palagin I, Rachina S, Sukhorukova M, Nizhegorodtseva I, Portnyagina U, Gordeeva S, Burasova E, Bagin V, Domanskaya O, Nathwani D, et al. Current Antimicrobial Stewardship Practice and Education in Russian Hospitals: Results of a Multicenter Survey. Antibiotics. 2021; 10(8):892. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10080892
Chicago/Turabian StylePalagin, Ivan, Svetlana Rachina, Marina Sukhorukova, Irina Nizhegorodtseva, Ulyana Portnyagina, Svetlana Gordeeva, Elena Burasova, Vladimir Bagin, Olga Domanskaya, Dilip Nathwani, and et al. 2021. "Current Antimicrobial Stewardship Practice and Education in Russian Hospitals: Results of a Multicenter Survey" Antibiotics 10, no. 8: 892. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10080892







