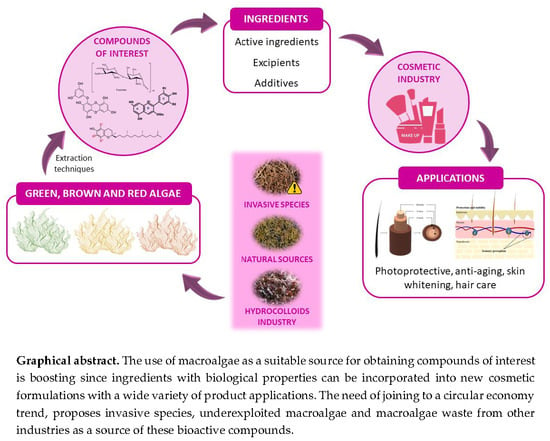
Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach
Abstract
:1. Perspectives of the Use of Macroalgae for Industrial Applications
2. Current Trends in the Cosmetic Industry and Its Main Objectives: Skin and Hair
3. Macroalgae-Based Metabolites of Interest for Cosmetics
3.1. Polysaccharides
3.2. Proteins and Amino Acids
3.3. Lipids
3.4. Pigments
3.4.1. Phycobiliproteins
3.4.2. Carotenoids
3.4.3. Chlorophylls
3.5. Phytohormones
3.6. Terpenoids and Halogenated Compounds
3.7. Phenolic Compounds: Polyphenols and Phlorotannins
3.8. Vitamins
4. Macroalgae Applications in Cosmetics
4.1. Active Ingredients
4.2. Excipients, Gelling and Thickening Agents
4.3. Additives
4.3.1. Preservatives
4.3.2. Dyes and Pigments
4.3.3. Aromas and Fragrances
5. Product Applications
5.1. Sunscreens: Photoprotection and Anti-Photoaging
5.2. Moisturizers
5.3. Anti-Aging Products
5.4. Skin Whitening
5.5. Haircare
5.6. Oral Care
5.7. Anti-Cellulite and Slimming Care
5.8. Peeling Products
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Spalding, H.L.; Amado-Filho, G.M.; Bahia, R.G.; Ballantine, D.L.; Fredericq, S.; Leichter, J.J.; Nelson, W.A.; Slattery, M.; Tsuda, R.T. Macroalgae. In Mesophotic Coral Ecosystems; Springer: New York, NY, USA, 2019; pp. 507–536. ISBN 978-3-319-92734-3. [Google Scholar]
- Pepper, I.L.; Gentry, T.J. Microorganisms Found in the Environment. In Environmental Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 9–36. ISBN 9780123946263. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Micro-Macroalgae Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 9780128035818. [Google Scholar]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem 2003, 2, 498–503. [Google Scholar]
- Silva, M.; Vieira, L.; Almeida, A.P.; Kijjoa, A. The Marine Macroalgae of the Genus Ulva: Chemistry, Biological Activities and Potential Applications. J. Oceanogr. Mar. Res. 2013, 1, 1–6. [Google Scholar]
- Mendes, M.; Pereira, R.; Sousa Pinto, I.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Stefan Sebök, W.H.; Hanelt, D. Development of an innovative ring-shaped cultivation system for a land-based cultivation of marine macroalgae. Aquac. Eng. 2017, 77, 33–41. [Google Scholar] [CrossRef]
- Vuong, D.; Kaplan, M.; Lacey, H.J.; Crombie, A.; Lacey, E.; Piggott, A.M. A study of the chemical diversity of macroalgae from South Eastern Australia. Fitoterapia 2018, 126, 53–64. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S. Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; pp. 346–369. ISBN 9780124080621. [Google Scholar]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards circular economy in the food system. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Moure, A.; Domínguez, H. Valorization of Sargassum muticum biomass according to the biorefinery concept. Mar. Drugs 2015, 13, 3745–3760. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Reddy, C.R.K. A simple process for recovery of a stream of products from marine macroalgal biomass. Bioresour. Technol. 2016, 203, 160–165. [Google Scholar] [CrossRef]
- Kuda, T.; Taniguchi, E.; Nishizawa, M.; Araki, Y. Fate of water-soluble polysaccharides in dried Chorda filum a brown alga during water washing. J. Food Compos. Anal. 2002, 15, 3–9. [Google Scholar] [CrossRef]
- Renner, G.; Audebert, F.; Burfeindt, J.; Calvet, B.; Caratas-Perifan, M.; Leal, M.E.; Gorni, R.; Long, A.; Meredith, E.; O’Sullivan, Ú.; et al. Cosmetics Europe guidelines on the management of undesirable effects and reporting of serious undesirable effects from cosmetics in the European Union. Cosmetics 2017, 4, 1. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Chapter 14—Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 423–441. ISBN 9780128027721. [Google Scholar]
- Etcoff, N.L.; Stock, S.; Haley, L.E.; Vickery, S.A.; House, D.M. Cosmetics as a Feature of the Extended Human Phenotype: Modulation of the Perception of Biologically Important Facial Signals. PLoS ONE 2011, 6, e25656. [Google Scholar] [CrossRef] [Green Version]
- Aurora, N.; Agarwal, S.; Murthy, R.S.R. Latest technology advances in cosmaceuticals. Int. J. Pharm. Sci. Drug Res. 2019, 4, 168–182. [Google Scholar]
- Wang, H.D.; Chen, C.; Huynh, P.; Chang, J. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2012, 184, 355–362. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chou, Y.-T.; Wen, Z.-H.; Wang, C.-Z.; Chen, C.-H.; Ho, M.-L. Correction: Novel Biodegradable Porous Scaffold Applied to Skin Regeneration. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Tataru, G.; Popa, M.; Costin, D.; Desbrieres, J. Microparticles based on natural and synthetic polymers for ophthalmic applications. J. Biomed. Mater. Res. 2012, 100A, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Brynjolfsdottir, A.; Krutmann, J. Pharmaceutical and Cosmetic USe of Extracts from Algae Obtanable from Saline Hot Water Sources. U.S. Patent PCT/IS2007/000012, 10 July 2010. [Google Scholar]
- Stuts, C.S.; Schmid, D.; Zülli, F. Use of an Extract from Snow Algae in Cosmetic Ordermatological Formulations. U.S. Patent US12/760,173, 12 September 2012. [Google Scholar]
- Asselineau, D.; Bernerd, F.; Fagot, D.; Pageon, H. Natural and photo-induced aging of the skin: The three dimensional culture approach. J. Soc. Biol. 2003, 197, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Luisa, M.; Nogueira, D.R.; González-lópez, N.; Conde, E.; Moure, A.; Pilar, M. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crops Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Park, A.M.; Khan, S.; Rawnsley, J. Hair Biology Growth and Pigmentation. Facial Plast. Surg. Clin. N. Am. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Mirmirani, P. Ceramic flat irons: Improper use leading to acquired trichorrhexis nodosa. J. Am. Dermatol. 2010, 62, 145–147. [Google Scholar] [CrossRef]
- Monselise, A.; Cohen, D.E.; Wanser, R.; Shapiro, J. What Ages Hair? Int. J. Womens Dermatol. 2017, 3 (Suppl. 1), S52–S57. [Google Scholar] [CrossRef]
- Sugiyama, Y. Polysaccharides. In Immunotherapy of Cancer: An Innovative Treatment Comes of Age; Springer: Tokyo, Japan, 2016; pp. 37–50. ISBN 9784431550310. [Google Scholar]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, Cosmeceutical, and Traditional Applications of Marine Carbohydrates, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 73, ISBN 9780128002681. [Google Scholar]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing Limited: Cambridge, UK, 2013; ISBN 9780857095121. [Google Scholar]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 489–532. [Google Scholar]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Sabalingam, S.; Jayasuriya, W.J.A.B.N. Pharmaceutical Excipients of Marine and Animal origin: A Review. Biol. Chem. Res. 2019, 6, 184–196. [Google Scholar]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Obluchinsksya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of Ultrasound Treatment on the Chemical Composition and Anticoagulant Properties of Dry Fucus Extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, L.; Şekeroğlu, N.; Kijjoa, A. The Potential of Marine Resources in Cosmetics. Curr. Perspect. Med. Aromat. Plants 2018, 1, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Bloch, J.F.; Tardieu-Guigues, E. Marine biotechnologies and synthetic biology, new issues for a fair and equitable profit-sharing commercial use. Mar. Genom. 2014, 17, 79–83. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, E. Lipids, Pharmaceutical and Cosmetic Use. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef]
- Sakthivel, R.; Pandima Devi, K. Evaluation of physicochemical properties, proximate and nutritional composition of Gracilaria edulis collected from Palk Bay. Food Chem. 2015, 174, 68–74. [Google Scholar] [CrossRef]
- Debbarma, J.; Madhusudana Rao, B.; Narasimha Murthy, L.; Mathew, S.; Venkateshwarlu, G.; Ravishankar, C.N. Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J. Fish. 2016, 63, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269–1278. [Google Scholar]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Maia Campos, P.M.B.G. In Vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Modern moisturizer myths, misconceptions, and truths. Cutis 2013, 91, 308–314. [Google Scholar]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 275–318. ISBN 9780128027936. [Google Scholar]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Rizzo, R.F.; dos Santos, B.d.N.C.; de Castro, G.F.P.d.S.; Passos, T.S.; Nascimento, M.d.A.; Guerra, H.D.; da Silva, C.G.; Dias, D.d.S.; Domingues, J.R.; de Lima-Araújo, K.G. Production of phycobiliproteins by Arthrospira platensis under different light conditions for application in food products. Food Sci. Technol. 2015, 35, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [Green Version]
- Pandey, V.D.; Pandey, A.; Sharma, V. Biotechnological applications of cyanobacterial phycobiliproteins. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 89–97. [Google Scholar]
- Reis, A.; Mendes, A.; Lobo-Fernandes, H.; Empis, J.A.; Novais, J.M. Production, extraction and purification of phycobiliproteins from Nostoc sp. Bioresour. Technol. 1998, 66, 181–187. [Google Scholar] [CrossRef]
- Sonani, R.R. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins: Valuable proteinic pigments in red seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 321–343. ISBN 9780124080621. [Google Scholar]
- Arad, S.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, T.; Mori, I.C.; Ikeda, Y.; Hirayama, T.; Mikami, K. Comprehensive phytohormone quantification in the red alga Pyropia yezoensis by liquid chromatography–mass spectrometry. In Protocols for Macroalgae Research; Francis & Taylor Group, CRC Press: Boca Raton, FL, USA, 2019; pp. 225–236. [Google Scholar]
- Michelet, J.F.; Olive, C.; Rieux, E.; Fagot, D.; Simonetti, L.; Galey, J.B.; Dalko-Csiba, M.; Bernard, B.A.; Pereira, R. The anti-ageing potential of a new jasmonic acid derivative (LR2412): In vitro evaluation using reconstructed epidermis episkinTM. Exp. Dermatol. 2012, 21, 398–400. [Google Scholar] [CrossRef]
- Jacinto, M.S.C.; Monteiro, H.R.; Lemos, M.F.L. Impact of the invasive macroalgae Asparagopsis armata on coastal environments: An ecotoxicological assessment. Curr. Opin. Biotechnol. 2013, 24, S75. [Google Scholar] [CrossRef]
- Lobo, A.M.; Lourenço, A.M. Biossíntese de Produtos Naturais; IST Presse: Lisbon, Portugal, 2007; ISBN 9789728469504. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Saraiva, J.A. Mutielemental concentration and physiological responses of Lavandula pedunculata growing in soils developed on different mine wastes. Environ. Pollut. 2016, 213, 43–52. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [Green Version]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Van Tran, V.; Loi Nguyen, T.; Moon, J.Y.; Lee, Y.C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2019, 368, 88–114. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Ogaji, I.J.; Nep, E.I.; Audu-Peter, J.D. Advances in Natural Polymers as Pharmaceutical Excipients. Pharm. Anal. Acta 2012, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bellion, C.; Brigand, G.; Prome, J.-C.; Bociek, D.W.S. Identification et Caractérisation des Précurseurs Biologiques des Carraghénanes par Spectroscopie de RMN C13. Carbohydr. Res. 1983, 119, 31–48. [Google Scholar] [CrossRef]
- Dea, I.C.M.; McKinnon, A.A.; Rees, D.A. Tertiary and Quaternary Structure in Aqueous Polysaccharide Systems which Model Cell Wall Cohesion: Reversible Changes in Conformation and Association of Agarose, Carrageenan and Galactomannans. J. Mol. Biol. 1972, 68, 153–172. [Google Scholar] [CrossRef]
- Renn, D. Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Jani, G.K.; Shah, D.P.; Prajapatia, V.D.; Jain, V.C. Gums and mucilages: Versatile excipients for pharmaceutical formulations. Asian J. Pharm. Sci. 2009, 4, 309–323. [Google Scholar]
- Nakano, M.; Kouketsu, M.; Nakamura, Y.; Juni, K. Sustained Release of Sulfamethizole from Agar Beads after Oral Administration to Humans. Chem. Pharm. Bull. 1980, 28, 2905–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Helw, A.E.R.; El-Said, Y. Preparation and characterization of agar beads containing phenobarbitone sodium. J. Microencapsul. 1988, 5, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Philip, A.K.; Pathak, K. Modified polysaccharides as fast disintegrating excipients for orodispersible tablets of roxithromycin. AAPS PharmSciTech 2008, 9, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [Green Version]
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast) (Text with EEA relevance). Off. J. Eur. Union 2009, 27, 152–302. [Google Scholar]
- Alvarez-Rivera, G.; Llompart, M.; Lores, M.; Garcia-Jares, C. Preservatives in Cosmetics: Regulatory Aspects and Analytical Methods. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 175–224. ISBN 9780444635167. [Google Scholar]
- Myers, E.; Pritchett, T.; Brettell, T. Determination of Preservatives in Cosmetics and Personal Care Products by LC–MS-MS. Lc Gc N. Am. 2015, 33, 16–22. [Google Scholar]
- Basketter, D.; White, I.R. Legislative aspects of cosmetic safety in the European Union: The case of contact allergy. Cosmetics 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Bernauer, U.; Chaudhry, Q.; Coenraads, P.J.; Degen, G.H.; Dusinska, M.; Lilienblum, W.; Nielsen, E.; Platzek, T.; Rousselle, C.H.; van Benthem, J.; et al. Opinion of the Scientific Committee on Consumer safety (SCCS)—Opinion on the safety of the use of Methylisothiazolinone (MI) (P94), in cosmetic products (sensitisation only). Regul. Toxicol. Pharmacol. 2016, 76, 211–212. [Google Scholar]
- Sasseville, D. Hypersensitivity to preservatives. Dermatol. Ther. 2004, 17, 251–263. [Google Scholar] [CrossRef]
- Schwensen, J.F.; White, I.R.; Thyssen, J.P.; Menné, T.; Johansen, J.D. Failures in risk assessment and risk management for cosmetic preservatives in Europe and the impact on public health. Contact Dermat. 2015, 73, 133–141. [Google Scholar] [CrossRef]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2014, 59, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in a aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El-Baroty, G.S. Natural preservative ingredient from marine alga Ulva lactuca L. Int. J. Food Sci. Technol. 2009, 44, 1688–1695. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Yoo, D.I.; Shin, Y. Preparation of Lip Balm Utilizing Functionalities of Colorants Extracted from Marine Algae. Text. Color. Finish. 2014, 26, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Salvador, N.; Gómez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Gago-Dominguez, M.; Castelao, J.E.; Yuan, J.M.; Yu, M.C.; Ross, R.K. Use of permanent hair dyes and bladder-cancer risk. Int. J. Cancer 2001, 91, 575–579. [Google Scholar] [CrossRef]
- Europena Commission. Commission Regulation (EU) No 1197/2013 of 25 November 2013 amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products 2013. Off. J. Eur. Union 2013, 315, 34–66. [Google Scholar]
- Europena Commission. Commission Regulation (EU) 2015/1298 of July 28 2015 Amending Annexes II and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; European Parliament, C. of the E.U., Ed.; European Parliament, Council of the European Union: Brussels, Belgium, 2015; Volume 199, pp. 22–23. [Google Scholar]
- European Commission. Commission Regulation (EU) No 1004/2014 of 18 September 2014 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2014, 282, 5–8. [Google Scholar]
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543. [Google Scholar] [CrossRef]
- Scher, R.K. Cosmetics and ancillary preparations for the care of nails: Composition, chemistry, and adverse reactions. J. Am. Acad. Dermatol. 1982, 6, 523–528. [Google Scholar] [CrossRef]
- Volpe, M.G.; Nazzaro, M.; Coppola, R.; Rapuano, F.; Aquino, R.P. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem. J. 2012, 101, 65–69. [Google Scholar] [CrossRef]
- Wenzel, S.M.; Welzel, J.; Hafner, C.; Landthaler, M.; Bäumler, W. Permanent make-up colorants may cause severe skin reactions. Contact Dermat. 2010, 63, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morancais, M.; Fleurence, J.; Tran, T.N.L.; Dumay, J. Extracting and Purifying Pigment R-phycoerythrin from the Red alga Mastocarpus Stellatus. In Proceedings of the 2018 4th International Conference on Green Technology and Sustainable Development, GTSD, Ho Chi Minh City, Vietnam, 23–24 December 2018; pp. 573–577. [Google Scholar]
- Sfriso, A.A.; Gallo, M.; Baldi, F. Phycoerythrin productivity and diversity from five red macroalgae. J. Appl. Phycol. 2018, 30, 2523–2531. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M.; Nguyen, H.P.T.; Fleurence, J. Extraction and purification of r-phycoerythrin from marine red algae. Methods Mol. Biol. 2015, 1308, 109–117. [Google Scholar]
- Wang, L.; Qu, Y.; Fu, X.; Zhao, M.; Wang, S.; Sun, L. Isolation, Purification and Properties of an R-Phycocyanin from the Phycobilisomes of a Marine Red Macroalga Polysiphonia urceolata. PLoS ONE 2014, 9, e101724. [Google Scholar] [CrossRef] [Green Version]
- Bayerl, C. Beta-carotene in dermatology: Does it help? Acta Derm. Alp Pannonica Adriat 2008, 17, 160–162, 164–166. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Mauro, M. Sensitization to fragrance mix-1 in patients with contact dermatitis in Nord-East of Italy: 1996-2016 time trend and gender effect. Cosmetics 2019, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, K.J.; Yiannias, J.A. Contact dermatitis to cosmetics, fragrances, and botanicals. Dermatol. Ther. 2004, 17, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Beauchêne, D.; Grua-Priol, J.; Lamer, T.; Demaimay, M.; Quémeneur, F. Concentration by pervaporation of aroma compounds from Fucus serratus. J. Chem. Technol. Biotechnol. 2000, 75, 451–458. [Google Scholar] [CrossRef]
- Harsha, L.; Brundha, M.P. Role of collagen in wound healing. Drug Invent. Today 2020, 13, 55–57. [Google Scholar]
- Sudel, K.M.; Venzke, K.; Mielke, H.; Breitenbach, U.; Mundt, C.; Jaspers, S.; Koop, U.; Sauermann, K.; Knussman-Hartig, E.; Moll, I.; et al. Novel aspects of intrinsic and extrinsic aging of human skin: Beneficial effects of soy extract. Photochem. Photobiol. 2005, 81, 581–587. [Google Scholar] [CrossRef]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys Siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.M.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Pavia, H.; Cervin, G.; Lindgren, A.; Aberg, P. Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 1997, 157, 139–146. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. Protective effect of chromene isolated from Sargassum horneri against UV-A-induced damage in skin dermal fibroblasts. Exp. Dermatol. 2012, 21, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective effect of the ethyl acetate fraction of Sargassum muticum against ultraviolet B-irradiated damage in human keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146–8160. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for skin hydrating cosmetics. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: New York, NY, USA, 2015; pp. 1867–1892. ISBN 9783319162980. [Google Scholar]
- Choi, J.S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.J.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet. Sci. 2013, 64, 193–205. [Google Scholar]
- Cai, C.E.; Yang, Y.Y.; Cao, R.D.; Jia, R.; He, P.M. Derivatives from Two Algae: Moisture Absorption-Retention Ability, Antioxidative and Uvioresistant Activity. J. Biobased Mater. Bioenergy 2018, 12, 277–282. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci 2014, 41, 1195–1207. [Google Scholar]
- Guinea, M.; Franco, V.; Araujo-Bazán, L.; Rodríguez-Martín, I.; González, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.C. The potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. South Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, S.-K.; Bak, S.-S. Hair Biology and Care Product Ingredients from Marine Organisms. In Marine Cosmeceuticals; CRC Press, Taylor&Francis Group: Boca Ratón, FL, USA, 2012; pp. 201–210. [Google Scholar]
- Bak, S.S.; Ahn, B.N.; Kim, J.A.; Shin, S.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K.; Kim, S.K. Ecklonia cava promotes hair growth. Clin. Exp. Dermatol. 2013, 38, 904–910. [Google Scholar] [CrossRef]
- Ahsan, H. The significance of complex polysaccharides in personal care formulations. J. Carbohydr. Chem. 2019, 38, 213–233. [Google Scholar] [CrossRef]
- Anil, S.; Venkatesan, J.; Chalisserry, E.P.; Nam, S.Y.; Kim, S.K. Applications of Seaweed Polysaccharides in Dentistry. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 331–340. ISBN 9780128098172. [Google Scholar]
- Choi, J.S.; Ha, Y.M.; Joo, C.U.; Cho, K.K.; Kim, S.J.; Choi, I.S. Inhibition of oral pathogens and collagenase activity by seaweed extracts. J. Environ. Biol. 2012, 33, 115–121. [Google Scholar]
- Fabrowska, J.; Łȩska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and Extracts of Algae as Material for Cosmetics. In Marine Algae Extracts: Processes, Products, and Applications; Wiley VCH: Weinheim, Germany, 2015; Volume 2, pp. 681–706. ISBN 9783527679577. [Google Scholar]
- Kozlowska, J.; Prus, W.; Stachowiak, N. Microparticles based on natural and synthetic polymers for cosmetic applications. Int. J. Biol. Macromol. 2019, 129, 952–956. [Google Scholar] [CrossRef]


| PS | Sources | Properties | Applications | Ref. |
|---|---|---|---|---|
| Brown macroalgae | ||||
| Alginates | Turbinaria conoides (J. Agardh) Kützing Macrocystis pyrifera (L.) C. Agardh, Sargassum horneri (Turner) C. Agardh | Free radical scavenging activity inhibition of inflammatory response and gel-forming activity. | Emulsifier and thickening agent | [39,40] |
| Fucans | Laminaria japonica Areschoug, Kjellmaniella crassifolia Miyabe, Fucus vesiculosus L., Sargassum binderi Sonder ex J. Agardh, T. conoides, Sargassum hemiphyllum (Turner) C. Agardh, Nemacystus decipiens (Suringar) Kuckuck, Cladosiphon okamuranus Tokida, Undaria pinnatifida (Harvey) Suringar, Eisenia spp., Ecklonia spp., Ascophyllum nodosum (L.) Le Jolis, Lessonia nigrescens Bory, Sargassum polycystum C. Agardh | Radical scavenging, antioxidant, anti-inflammatory, anticoagulant, antiviral, antithrombotic, and anticancer and UV protection | Active ingredients, photoprotection, skin-whitening and antiaging products | [41] |
| Laminarin | Eisenia bicyclis (Kjellman) Setchell Laminariaceae family Ascophyllum, Undaria, Fucus genera, | Wound-healing, immunomodulatory, antitumor, scavenging, antibacterial, antioxidant activity. Modulatory effects on skin cells, hydro-gelling properties | Active ingredients, photoprotection and antiaging products | [42] |
| Red macroalgae | ||||
| Carrageenans | Kappaphycus and Eucheuma genera fundamentally | Antioxidant, antibacterial and immunomodulatory effects | Gel-forming, emulsifying, thickening, and stabilizing agent | [43] |
| Agar | Gelidium amansii (J.V. Lamouroux) J.V. Lamouroux | Scavenger properties, antioxidant and gel-forming activity | Thickening agent | [39,40] |
| Porphyran | Porphyra tenera Kjellman, Porphyra yezoensis Ueda and P. haitanensis T. J. Chang and B. F. Zheng | Antioxidant, anticancer, immuno-modulating, hypercholesterolemic, antihyperlipidemic, anti-inflammatory effects | Active ingredients and skin whitening products | [37] |
| Green macroalgae | ||||
| Ulvans | Ulva and Enteromorpha genera cell walls | Gel-forming, skin protective and antioxidant properties | Skin aging products | [44] |
| Xylans and mannans | Bryopsidales order | Gel-forming and other biological properties | Emulsifier and thickener | [45] |
| Type | Species | Lipids and Lipophilic Molecules | Properties |
|---|---|---|---|
| Brown macroalgae | A. nodosum | Fucoxanthin | Anti-aging agent, anti-wrinkle agent, smoothing agent |
| U. pinnatifida | Fucosterol, polyunsaturated fatty acids | Antioxidant and anticancer activities, UV protection, anti-aging agent, moisturizing properties | |
| Red macroalgae | C. crispus | Omega-3 and omega-6 | Emollient, moisturizing, sheaths damaged or dry hair, nutritive, skin-soothing, anti-inflammatory |
| Porphyra umbilicalis Kützing | α-Linolenic acid | Skin-conditioning agent | |
| Gracilaria edulis (S. G. Gmelin) P. C. Silva | Palmitic, linolenic, and oleic acids, Vitamins A, D2, K1 and E | Anti-aging agent, moisturizing properties | |
| Green macroalgae | Ulva lactuca L. | Oleic, linoleic, and linolenic acid, Vitamins D2, K1 and E | Antioxidant, anti-inflammatory, skin elasticity, collagen synthesis, anti-wrinkle, emollient, moisturizing |
| Compound | Properties | Applications | Ref. |
|---|---|---|---|
| Mycosporine-like amino acids (MAAs) | Low molecular weight, hydrosoluble and stable molecules. | Sunscreen ingredient. | [37,55] |
| Arginine | Precursor of urea. | Cosmetic formulations. | [56] |
| Phycobiliproteins | Stable at pH ranges of 5–9. Antioxidant, antidiabetic, anti-hypertensive, immune-modulator, anti-inflammatory, anti-aging. | Reduce the use of synthetic colorants. Dyes in eye shadows, makeup, creams or lipsticks. | [74,75,76] |
| Zeaxanthin | Anti-tyrosinase activity. | Prevention of skin spots formation. Whitening agent. | [23,77] |
| Fucoxanthin | Reduction of: ROS, MMPs expression, (MMP13, related to tumor processes) and tyrosinase activity. | Oral or cutaneous administration. Inhibition of melanogenesis. | [23,67] |
| Pheophytin a and pheophorbide a | Antioxidant bioactivity. | Cosmetic industry as antioxidants. | [78] |
| Chromene meroterpenoid and tetraprenyltoluquinol | Reduction of ROS by 21%. Anti-photo-oxidative stress activity. | Sunscreen ingredient. | [67] |
| Spatane diterpenoids | Anti-tumor capacity. | Creams, sunscreens and other products. | [67] |
| Halogenated compounds derived from Iodine | Promoting lipolysis (thyroid metabolism). | Cosmetic industry. | [23] |
| Bromoform and dibromoacetic acid | Antimicrobial properties. | Preservatives. | [82] |
| Eckstolonol | Reduction of ROS. Increment in enzymatic activity. Decrease of protein levels, pro-apoptotic factors (p53, Bax) and caspases 3 and 9. Antioxidant and photo-protective properties. | Anticancer therapies. Sunscreens. | [32,67] |
| Phloroeckol and phloroglucinol | Anti-diabetic and antioxidant properties. | Anti-skin aging products. | [77] |
| Phenolic compounds and other phlorotannins | Inhibiting the MMP overexpression. Photoprotective properties. | Prevention of collagen premature degradation, wrinkle formation and the appearance of cancer cells. | [6] |
| Vitamins A and E | Protective effects against DNA damage. Ability to favor cell renewal. | Sunscreens. | [6] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/resources9090101
Lourenço-Lopes C, Fraga-Corral M, Jimenez-Lopez C, Pereira AG, Garcia-Oliveira P, Carpena M, Prieto MA, Simal-Gandara J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources. 2020; 9(9):101. https://0-doi-org.brum.beds.ac.uk/10.3390/resources9090101
Chicago/Turabian StyleLourenço-Lopes, Catarina, Maria Fraga-Corral, Cecilia Jimenez-Lopez, Antia G. Pereira, Paula Garcia-Oliveira, Maria Carpena, Miguel A. Prieto, and Jesus Simal-Gandara. 2020. "Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach" Resources 9, no. 9: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/resources9090101