Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Drug, Antibodies, Plasmids, and Small Interfering RNA (siRNA)
2.3. Cell Proliferation Assay
2.4. Cell Migration and Invasion Assays
2.5. Gene Expression Profiling
2.6. Quantitative Real-Time Reverse Transcription (RT) PCR
2.7. Transfection and Western Blotting Analysis
2.8. Animals
2.9. Xenograft Assays and Drug Administration
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
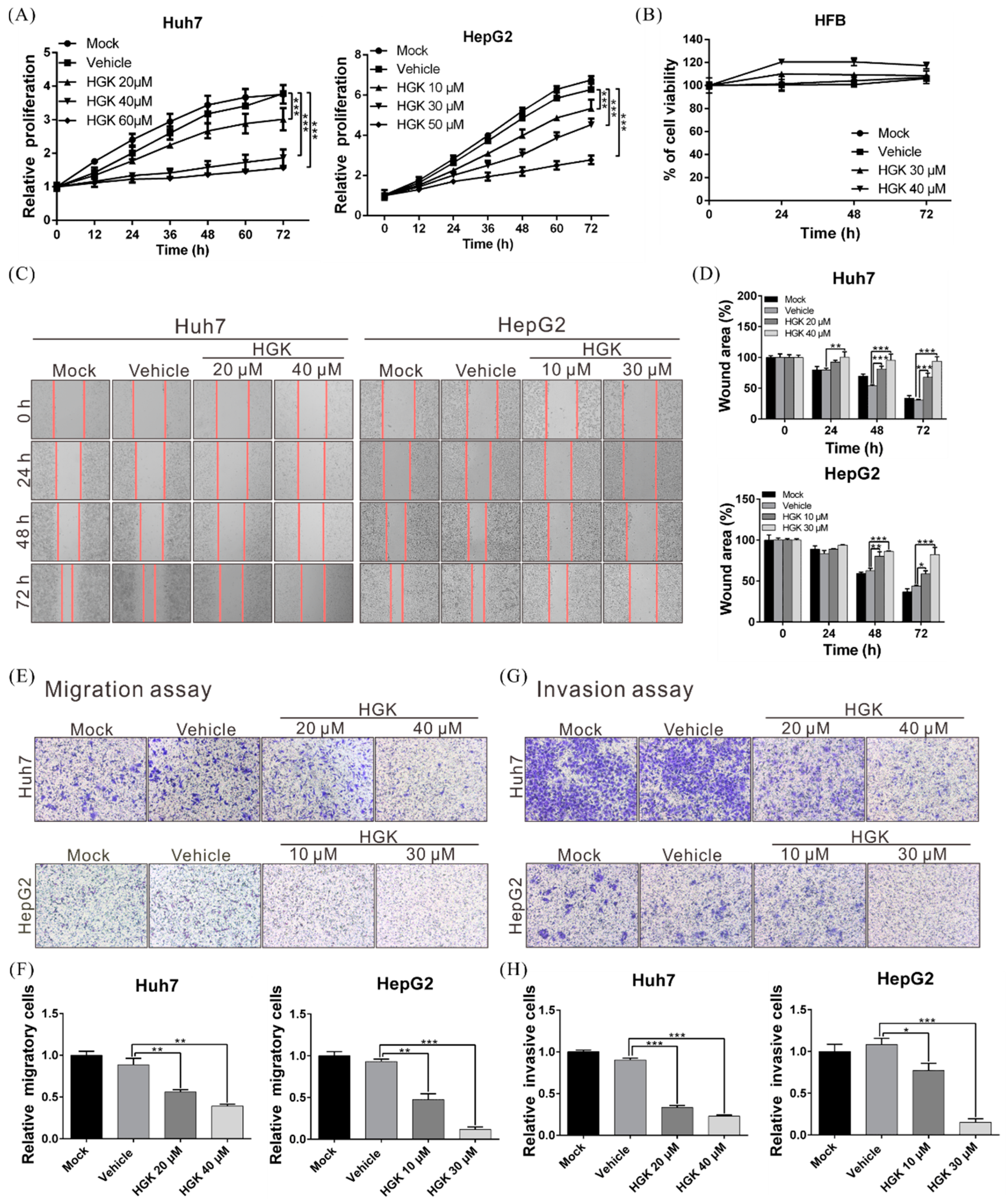
3.1. HGK Suppresses the Proliferation, Migration, and Invasion of Liver Cancer Cells
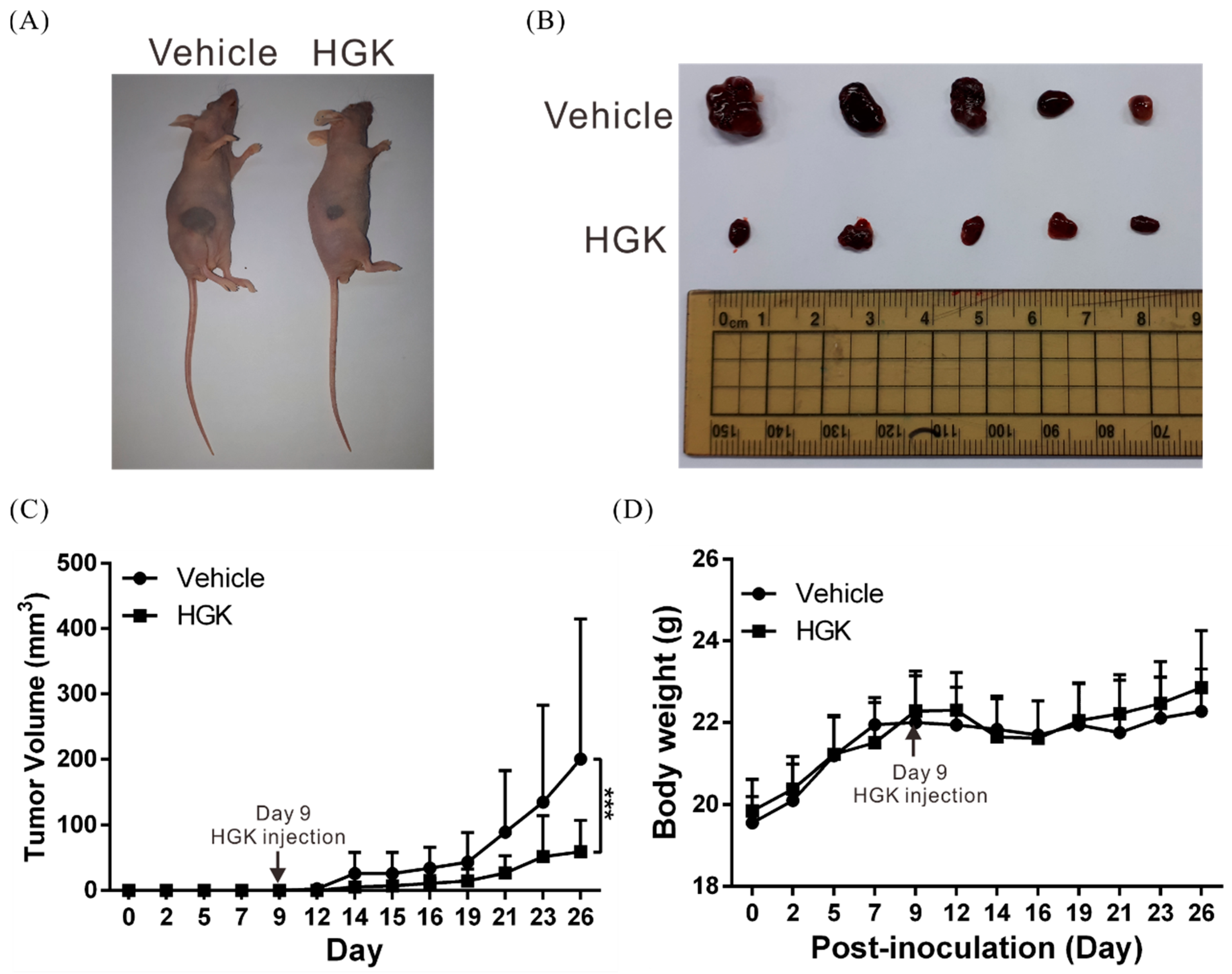
3.2. HGK Suppresses Tumor Growth in Mice Without Causing Physiological Toxicity
3.3. HGK Inhibits the Expression of the Transcription Factor FOXM1
3.4. HGK Suppresses the Expression of EMT-Related Genes by Inhibiting FOXM1 Expression
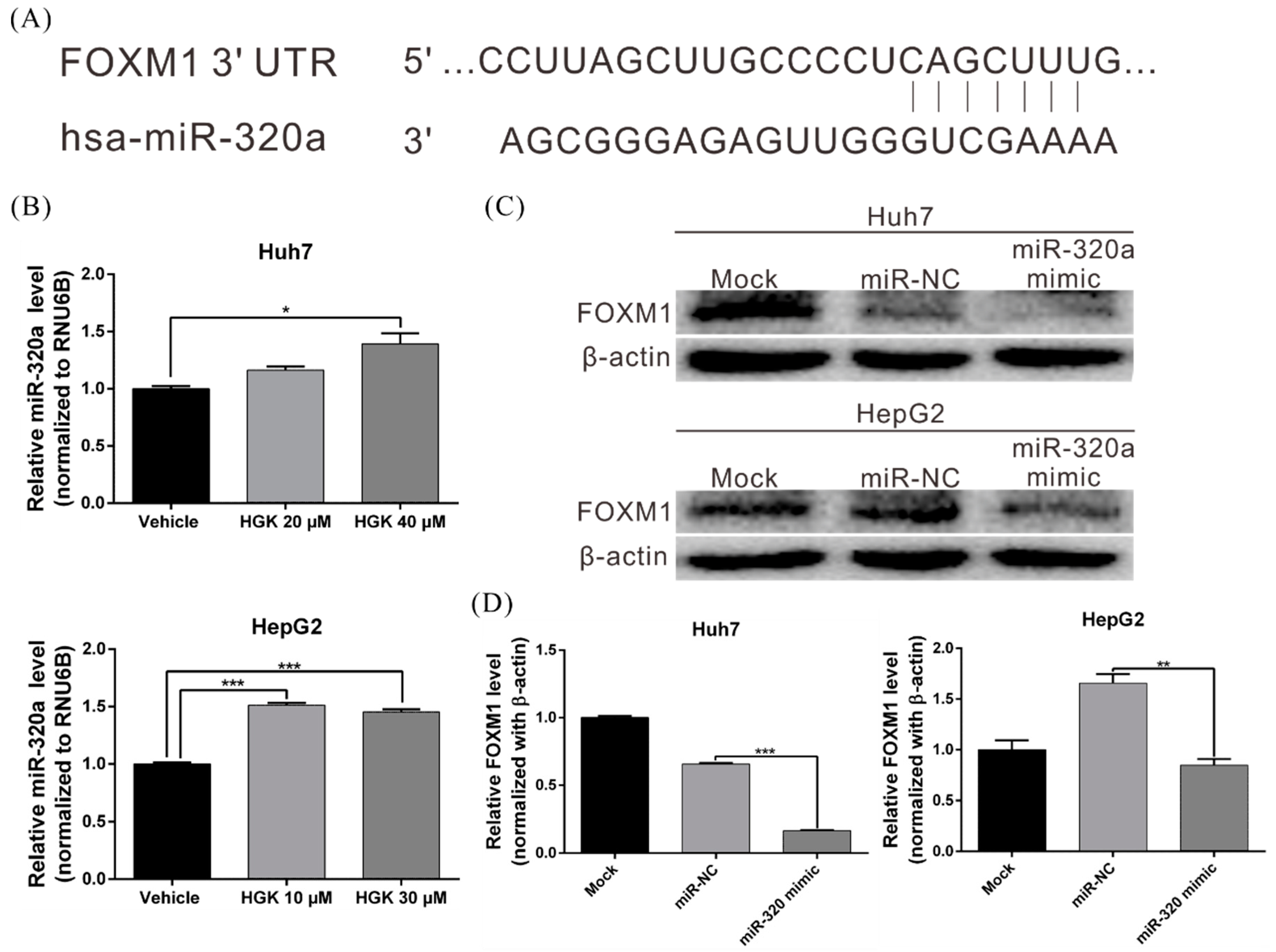
3.5. HGK Inhibits FOXM1 Expression by Inducing miR-320a Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Jin, R.; Zhao, J.; Liu, J.; Ying, H.; Yan, H.; Zhou, S.; Liang, Y.; Huang, D.; Liang, X.; et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015, 367, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef]
- Iavarone, M.; Colombo, M. HBV infection and hepatocellular carcinoma. Clin. Liver Dis. 2013, 17, 375–397. [Google Scholar] [CrossRef]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- Xie, L.; Yin, J.; Xia, R.; Zhuang, G. Cost-effectiveness of antiviral treatment after resection in hepatitis B virus-related hepatocellular carcinoma patients with compensated cirrhosis. Hepatology 2018, 68, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Huang, W.; Tian, D.; Zhu, H.; Zhang, Y.; Hu, H.; Fan, D.; Nie, Y.; Wu, K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J. Hepatol. 2012, 57, 600–612. [Google Scholar] [CrossRef]
- Sung, W.K.; Zheng, H.; Li, S.; Chen, R.; Liu, X.; Li, Y.; Lee, N.P.; Lee, W.H.; Ariyaratne, P.N.; Tennakoon, C.; et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 2012, 44, 765–769. [Google Scholar] [CrossRef]
- Ding, D.; Lou, X.; Hua, D.; Yu, W.; Li, L.; Wang, J.; Gao, F.; Zhao, N.; Ren, G.; Li, L.; et al. Recurrent targeted genes of hepatitis B virus in the liver cancer genomes identified by a next-generation sequencing-based approach. PLoS Genet. 2012, 8, e1003065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, P.; Xu, J.; Yu, Y.; Winkler, C.A. Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front. Genet. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Ferrone, C. Hepatocellular carcinoma surgical therapy: Perspectives on the current limits to resection. Chin. Clin. Oncol. 2018, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.E.; Bolonesi, R.M.; Rashid, A.; Elsayes, K.M.; Elbanan, M.G.; Law, L.; Kaseb, A.; Shroff, R.T. Systemic therapy for unresectable, mixed hepatocellular-cholangiocarcinoma: Treatment of a rare malignancy. J. Gastrointest Oncol. 2017, 8, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Zhu, A.X.; Farah, W.; Almasri, J.; Zaiem, F.; Prokop, L.J.; Murad, M.H.; Mohammed, K. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2018, 67, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Worns, M.A.; Galle, P.R. HCC therapies--lessons learned. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 447–452. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. WJG 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Wang, N.; Tsao, S.W.; Feng, Y. Current Status of Herbal Medicines in Chronic Liver Disease Therapy: The Biological Effects, Molecular Targets and Future Prospects. Int. J. Mol. Sci. 2015, 16, 28705–28745. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, G.; Han, Y.; Xu, J.; Gong, S.; Li, Y.; Zhang, H.; Liu, C. The method of quality marker research and quality evaluation of traditional Chinese medicine based on drug properties and effect characteristics. Phytomedicine 2018, 44, 204–211. [Google Scholar] [CrossRef]
- Houh, Y.K.; Kim, K.E.; Park, S.; Hur, D.Y.; Kim, S.; Kim, D.; Bang, S.I.; Yang, Y.; Park, H.J.; Cho, D. The Effects of Artemisinin on the Cytolytic Activity of Natural Killer (NK) Cells. Int. J. Mol. Sci. 2017, 18, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.X.; Li, Y.; Yin, H.; Zhang, J. Curcumin: Updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012, 13, 3959–3978. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Tan, H.Y.; Li, S.; Cheung, F.; Wang, N.; Nagamatsu, T.; Feng, Y. Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds. Int. J. Mol. Sci. 2016, 17, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlikowska, K.M.; Witkowska, A.M.; Zujko, M.E.; Dobrzycka, B.; Terlikowski, S.J. Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int. J. Mol. Sci. 2014, 15, 21703–21722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [PubMed] [Green Version]
- Li, S.; Chou, G.; Hseu, Y.; Yang, H.; Kwan, H.; Yu, Z. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem. Cent. J. 2013, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.L.; Zhang, D.D. Anti-inflammatory effects of 81 chinese herb extracts and their correlation with the characteristics of traditional chinese medicine. Evid. Based Complement Altern. Med. 2014, 2014, 985176. [Google Scholar] [CrossRef]
- Li, Y.N.; Yin, L.H.; Xu, L.N.; Peng, J.Y. A simple and efficient protocol for large-scale preparation of three flavonoids from the flower of Daphne genkwa by combination of macroporous resin and counter-current chromatography. J. Sep. Sci. 2010, 33, 2168–2175. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, R.R.; Huang, L.L.; Zhong, L.Q.; Yuan, S.T. [Studies on chemical constituents of Daphne genkwa]. Zhong Yao Cai 2009, 32, 508–511. [Google Scholar]
- Wang, Y.; Xu, Y.S.; Yin, L.H.; Xu, L.N.; Peng, J.Y.; Zhou, H.; Kang, W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chem. Biol. Interact. 2013, 206, 346–355. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Lin, Y.S.; Chen, Y.; Yeh, C.T.; Huang, Y.L.; Hsieh, T.H.; Shieh, T.M.; Hsueh, C.; Chen, T.C. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget 2015, 6, 23342–23357. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.C.; Shieh, T.M.; Hsueh, C.; Wang, S.H.; Leu, Y.L.; Lian, J.H.; Wang, T.H. Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5. Int. J. Mol. Sci. 2018, 19, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Chen, C.Y.; Wang, S.H.; Yeh, C.T.; Su, S.C.; Ueng, S.H.; Chuang, W.Y.; Hsueh, C.; Wang, T.H. Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers 2018, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Niu, Y.; Huang, C. Role of FoxM1 in the Progression and Epithelial to Mesenchymal Transition of Gastrointestinal Cancer. Recent Pat Anticancer Drug Discov. 2017, 12, 247–259. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Pan, J.; Geng, X.; Li, L.; Wu, J.; Song, P.; Wang, Y.; Liu, J.; Wang, L. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget 2016, 7, 29275–29286. [Google Scholar]
- Sun, J.Y.; Zhao, Z.W.; Li, W.M.; Yang, G.; Jing, P.Y.; Li, P.; Dang, H.Z.; Chen, Z.; Zhou, Y.A.; Li, X.F. Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of FOXM1 expression. Oncotarget 2017, 8, 61499–61509. [Google Scholar] [CrossRef]
- Gutman, J.; Kovacs, S.; Dorsey, G.; Stergachis, A.; Ter Kuile, F.O. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [Green Version]
- Bella, L.; Zona, S.; Nestal de Moraes, G.; Lam, E.W. FOXM1: A key oncofoetal transcription factor in health and disease. Semin Cancer Biol. 2014, 29, 32–39. [Google Scholar] [CrossRef]
- Zona, S.; Bella, L.; Burton, M.J.; Nestal de Moraes, G.; Lam, E.W. FOXM1: An emerging master regulator of DNA damage response and genotoxic agent resistance. Biochim. Biophys Acta 2014, 1839, 1316–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartel, A.L. FOXM1 in Cancer: Interactions and Vulnerabilities. Cancer Res. 2017, 77, 3135–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wen, L.; Wen, M.; Liu, T.; Zhao, L.; Wu, B.; Yun, Y.; Liu, W.; Wang, H.; Wang, Y.; et al. FoxM1 promotes epithelial-mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-Met/AKT-dependent positive feedback loop. Anticancer Drugs 2018, 29, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milewski, D.; Balli, D.; Ustiyan, V.; Le, T.; Dienemann, H.; Warth, A.; Breuhahn, K.; Whitsett, J.A.; Kalinichenko, V.V.; Kalin, T.V. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017, 13, e1007097. [Google Scholar] [CrossRef] [Green Version]
- Kopanja, D.; Pandey, A.; Kiefer, M.; Wang, Z.; Chandan, N.; Carr, J.R.; Franks, R.; Yu, D.Y.; Guzman, G.; Maker, A.; et al. Essential roles of FoxM1 in Ras-induced liver cancer progression and in cancer cells with stem cell features. J. Hepatol. 2015, 63, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Siraj, A.K.; Pratheeshkumar, P.; Parvathareddy, S.K.; Qadri, Z.; Thangavel, S.; Ahmed, S.; Al-Dayel, F.; Tulbah, A.; Ajarim, D.; Al-Kuraya, K.S. FoxM1 is an independent poor prognostic marker and therapeutic target for advanced Middle Eastern breast cancer. Oncotarget 2018, 9, 17466–17482. [Google Scholar] [CrossRef] [Green Version]
- Rajamanickam, S.; Panneerdoss, S.; Gorthi, A.; Timilsina, S.; Onyeagucha, B.; Kovalskyy, D.; Ivanov, D.; Hanes, M.A.; Vadlamudi, R.K.; Chen, Y.; et al. Inhibition of FoxM1-Mediated DNA Repair by Imipramine Blue Suppresses Breast Cancer Growth and Metastasis. Clin. Cancer Res. 2016, 22, 3524–3536. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ye, L.; Lei, Y.; Wan, J.; Chen, H. Downregulation of FoxM1 sensitizes nasopharyngeal carcinoma cells to cisplatin via inhibition of MRN-ATM-mediated DNA repair. BMB Rep. 2019, 52, 208–213. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, L.-F.; Chen, C.-Y.; Yang, W.-H.; Chen, C.-C.; Chang, J.-L.; Leu, Y.-L.; Liou, M.-J.; Wang, T.-H. Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression. Biomolecules 2020, 10, 20. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010020
Chou L-F, Chen C-Y, Yang W-H, Chen C-C, Chang J-L, Leu Y-L, Liou M-J, Wang T-H. Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression. Biomolecules. 2020; 10(1):20. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010020
Chicago/Turabian StyleChou, Li-Fang, Chi-Yuan Chen, Wan-Hua Yang, Chin-Chuan Chen, Junn-Liang Chang, Yann-Lii Leu, Miaw-Jene Liou, and Tong-Hong Wang. 2020. "Suppression of Hepatocellular Carcinoma Progression through FOXM1 and EMT Inhibition via Hydroxygenkwanin-Induced miR-320a Expression" Biomolecules 10, no. 1: 20. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10010020




