Microwave Absorption of Barium Borosilicate, Zinc Borate, Fe-Doped Alumino-Phosphate Glasses and Its Raw Materials
Abstract
:1. Introduction
2. Experimental Section
| Barium Boro Silicate (BBS) Glass (Wt.%) | Zinc Borate (ZBR) Glass (mol%) | Fe-doped Alumio Phosphate (FAP) Glass (in wt.%) | |
|---|---|---|---|
| Glass Batch Composition | SiO2 (46.50%), B2O3 (26.50%) Na2O (16.50%), BaO (10.50%) | B2O3 (65%), Al2O3 (5%), Na2O (5%), ZnO (25%) | P2O5 (77.6%), Al2O3 (14%), B2O3 (1.65%), MgO (2.25%), ZnO (4.50%) with Fe (2.67%), Sn(1.0%) as dopant |
| Raw material sources | SiO2 (from BremthalerQuarzitwerk GmbH (BQW), Germany), H3BO3 (Merck, Darmstadt, Germany), Na2CO3 (Recens Chemical 98% pure), BaCO3 (BDH lab 99.5% pure) | H3BO3 (Merck, Darmstadt, Germany), Na2CO3 (Merck, Darmstadt, Germany, 99.9% pure), Al2O3 (Merck, Darmstadt, Germany) and ZnO (Merck, Darmstadt, Germany, purity ≥ 99.0) | Al(PO3)3, BPO4 and Mg(PO3)2 (Merck 99.5% pure), ZnO (Merck 99.5%) and Fe metal powder and Sn metal powder (LOBA Chemie 99.5%) |
3. Results and Discussion
3.1. Microwave Absorption of Raw Material
3.1.1. Dielectric Loss and MW Absorption by SiO2


3.1.2. MW absorption of BPO4

3.1.3. MW Absorption of Meta-Phosphate Raw Material
Mg(PO3)2

Al(PO3)3

3.1.4. Al(OH)3

3.1.5. Na2CO3

3.1.6. BaCO3

3.1.7. H3BO3
3.2. Dielectric Loss and MW Absorption of Glass
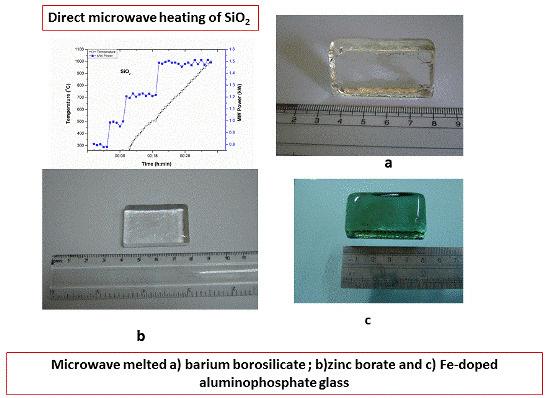
3.2.1. Barium Borosilicate Glass


3.2.2. Zinc Borate Glass (ZBR)


3.2.3. Fe-Doped Phosphate Glass


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sutton, W.H. Microwave processing of ceramic materials. Cer. Bull. 1989, 68, 376–386. [Google Scholar]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and application. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Shelbey, J.E. Introduction to Glass Science and Technology, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2005; pp. 27–30. [Google Scholar]
- Chenu, S.; Rocherullè, J.; Lebullenger, R.; Merdrignac, O.; Chevire, F.; Tessier, F.; Oudadesse, H. Synthesis and characterization of tin containing molybdophosphate and tungstophosphate glasses. J. Non-Cryst. Solids 2010, 356, 87–92. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Ganguli, M.; Rao, K.J. Novel method of preparation of inorganic glasses by microwave irradiation. J. Solid State Chem. 1994, 113, 448–450. [Google Scholar] [CrossRef]
- Ghussn, L.; Martinelli, J.L. A novel method to produce niobium phosphate glasses by microwave heating. J. Mater. Sci. 2004, 39, 1371–1376. [Google Scholar] [CrossRef]
- Duval, D.J.; Phillips, B.L.; Terjak, M.J.E.; Risbud, S.H. Reversible color changes and structural implications of microwave melting ion-conducting glasses. J. Solid State Chem. 1997, 131, 173–176. [Google Scholar] [CrossRef]
- Almeida, F.J.M.; Martinelli, J.R.; Partiti, C.S.M. Characterization of iron phosphate glasses prepared by microwave heating. J. Non-Cryst. Solids 2007, 353, 4783–4791. [Google Scholar] [CrossRef]
- Wang, J.S.; Jeng, J.S.; Ni, C.T. The study on the phosphate glass melted by microwave irradiation. J. Non-Cryst. Solids 2009, 355, 780–784. [Google Scholar] [CrossRef]
- Vaidhyanathan, B.; Rao, K.J. High microwave susceptibility of NaH2PO4.2H2O: Rapid synthesis of crystalline and glassy phosphates with NASICON-Type chemistry. J. Solid State Chem. 1997, 132, 349–354. [Google Scholar] [CrossRef]
- Hémono, N.; Chenu, S.; Lebullenger, R.; Rocherullé, J.; Kéryvin, V.; Wattiaux, A. Microwave synthesis and physical characterization of tin(II) phosphate glasses. J. Mater Sci. 2010, 45, 2916–2920. [Google Scholar] [CrossRef]
- Mandal, A.K.; Sinha, P.K.; Das, D.; Guha, C.; Sen, R. Higher Fe2+/total Fe ratio in Iron doped phosphate glass melted by microwave heating. Mater. Res. Bull. 2015, 63, 141–146. [Google Scholar] [CrossRef]
- Mandal, A.K.; Biswas, K.; Annapurna, K.; Guha, C.; Sen, R. Preparation of alumino-phosphate glass by microwave radiation. J. Mater. Res. 2013, 28, 1955–1961. [Google Scholar] [CrossRef]
- Mandal, A.K.; Balaji, S.; Sen, R. Microwave and conventional preparation of Zinc Borate glass: Eu3+ ion as luminescent probe. J. Alloys Compd. 2014, 615, 283–289. [Google Scholar] [CrossRef]
- Mandal, A.K.; Agrawal, D.; Sen, R. Preparation of homogeneous barium borosilicate glass using microwave energy. J. Non-Cryst. Solids 2013, 371–372, 41–46. [Google Scholar] [CrossRef]
- Peñaranda-Foix, F.L.; Janezic, M.D.; Civera, J.M.C.; Canós, A.J. Full-wave analysis of dielectric-loaded cylindrical waveguides and cavities using a new four-port ring network. IEEE T Microw. Theory 2012, 60, 2730–2740. [Google Scholar] [CrossRef]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of Inorganic Solids Using Microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Mandal, A.K.; Sen, S.; Mandal, S.; Guha, C.; Sen, R. Energy efficient melting of glass for nuclear waste immobilization using microwave radiation. Int. J. Green Energy 2014. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Mondal, A.; Upadhyaya, A.; Agrawal, D. Microwave sintering of W-18Cu and W-7Ni3Cu alloys. J. Microw. Power Electromagn. Energy 2009, 43, 11–16. [Google Scholar] [PubMed]
- Wang, L.; Wu, H.; Shen, Z.; Guo, S.; Wang, Y. Enhanced microwave absorption properties of Ni-doped ordered mesoporouscarbon/polyaniline nanocomposites. Mater. Sci. Eng. B 2012, 177, 1649–1654. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, L.D.; Wang, Y.M.; Guo, S.L.; Shen, Z.Y. Enhanced microwave absorbing properties of carbonyl iron-doped Ag/ordered mesoporous carbon nanocomposites. Mater. Sci. Eng. B 2012, 177, 476–482. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.K.; Sen, R. Microwave Absorption of Barium Borosilicate, Zinc Borate, Fe-Doped Alumino-Phosphate Glasses and Its Raw Materials. Technologies 2015, 3, 111-125. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies3020111
Mandal AK, Sen R. Microwave Absorption of Barium Borosilicate, Zinc Borate, Fe-Doped Alumino-Phosphate Glasses and Its Raw Materials. Technologies. 2015; 3(2):111-125. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies3020111
Chicago/Turabian StyleMandal, Ashis Kumar, and Ranjan Sen. 2015. "Microwave Absorption of Barium Borosilicate, Zinc Borate, Fe-Doped Alumino-Phosphate Glasses and Its Raw Materials" Technologies 3, no. 2: 111-125. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies3020111