Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges
Abstract
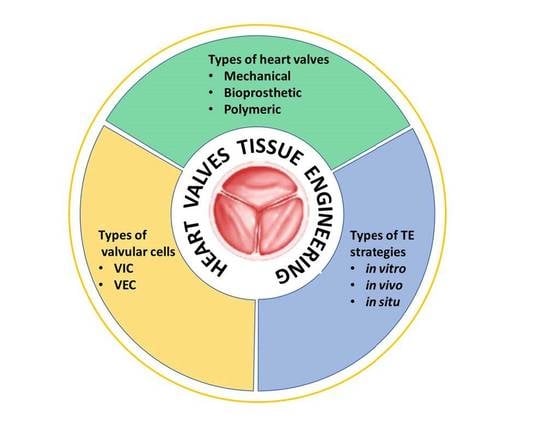
:1. Introduction
2. Scaffolds for Tissue Engineering: General Concepts
- The produced scaffold should have adequate mechanical properties, to mimic the anatomical site where it is intended to be implanted, and to function from the time of implantation to the completion of the remodeling process [55]. A scaffold’s mechanical properties (strength, modulus, toughness and ductility) are determined both by the material properties of the bulk material and by its structure (macrostructure, microstructure and nanostructure). Matching the mechanical properties of a scaffold to the graft is critically important, so that the progression of tissue healing is not limited by its mechanical failure prior to complete tissue regeneration [56]. Many materials have been produced with good mechanical properties, but to the detriment of retaining high porosity. In addition, many of these materials, with demonstrated in vitro potential, have failed when they were implanted in vivo because of insufficient capacity of vascularization [53]. Thus, to achieve a suitable scaffold, it is necessary to balance the mechanical properties with a porous structure, sufficient to allow cell infiltration and vascularization.
- Interface adherence of the scaffold referred to the interactions between cells and their environment, which play a critical role in determining cell fate and physiological functions, so as to maintain normal phenotypic shape within the scaffold. An ideal scaffold should provide informative microenvironments mimicking physiological niches to direct advanced cell behaviors, such as differentiation, proliferation and apoptosis, without inducing pathological outcomes, such as calcification [4].
- The scaffold’s biocompatibility is related to the cell’s adherence, which should function normally, migrate onto the surface or even through the scaffold, begin to proliferate and, finally, have a negligible immune reaction. Thus, to be accepted in vivo, the host immune response should be minimal for the scaffold. The biocompatibility of the cross-linking agent used is particularly important, especially in cases where reactive groups of the cross-linker are incorporated into the hydrogel network and might then be released upon degradation. Although unreacted chemicals are usually eliminated after cross-linking through extensive washing in distilled water, as a rule, toxic cross-linkers should be avoided, in order to preserve the biocompatibility of the final scaffold [53].
- A scaffold should be biodegradable and the degradation products should be non-toxic and able to be eliminated from the body without interference with other organs. There are different mechanisms for in vivo degradation, such as hydrolysis, oxidation, enzymatic and physical degradation [57]. The biodegradation process permits to the cells to produce their own extracellular matrix and finally to replace the implanted or tissue-engineered constructed scaffolds, eliminating the need for further surgery to remove it. The scaffold’s degradation rate should be adjusted to match the rate of tissue regeneration so that it has disappeared completely once the tissue is repaired [58,59].
3. Heart Valve Replacements
3.1. Mechanical Valves
3.2. Bioprosthetic Valves
- The balloon-expandable valve (Edwards SAPIEN) was further subjected to an anticalcification process, consisting of GA fixation and a phospholipid extraction, and an additional “mildheat” treatment, which removes the unstable GA molecules;
- The self-expandable valve (Medtronic Corevalve) was initially made of bovine pericardium, but was later developed with a lower profile device, by using a more flared outflow design and a porcine pericardium.
3.3. Polymeric Valves
4. Heart Valve Tissue Engineering: Cells and Strategies
- -
- The fibrosa layer is located near the outflow surface and is made of collagen (COL) and represents densely aligned fibers that ensure the primary strength of the valves;
- -
- The ventricularis layer is located on the opposite surface of the entrance and is made of elastin (EL), with an important role in stretching and retraction during the cardiac cycle;
- -
- The spongiosa layer is located between the two layers mentioned above and is made of proteoglycans (PG)—glycosaminoglycans (GAG), with the role of loose connective tissue to facilitate the relative movements of the adjacent layers.
- (Myo)fibroblasts isolated from harvested vascular or dermal tissues;
- Mesenchymal stromal cells (MSCs) from bone marrow or adipose tissue;
- Prenatal or early postnatal sources of MSCs, where cells are harvested before or immediately after birth and used toward the synthesis of autologous valve tissue for replacement in early childhood;
- Amniotic membrane sources of MSCs (AM-MSCs);
- Amniotic fluid sources of MSCs (AF-MSCs);
- Chorionic villi sources of MSCs (CV-MSCs);
- Umbilical cord sources of MSCs (UC-MSCs), from the cord blood, Wharton’s jelly or perivascular tissue;
- Stem cell (iPSC)-derived endocardial cells with the potential to provide VIC-like cells by undergoing endothelial-to-mesenchymal transition, with the best potential to obtain the native VICs population, compared to other mesenchymal cells.
5. Natural Polymer-Based Scaffolds for Heart Valve Tissue Engineering
5.1. Polysaccharide-Based Scaffolds for Heart Valve Tissue Engineering
5.1.1. Chitosan-Based Scaffolds
5.1.2. Hyaluronic Acid-Based Scaffolds
5.1.3. Cellulose-Based Scaffolds
5.1.4. Alginate-Based Scaffolds
5.2. Protein-Based Materials as Scaffolds in Heart Valve Tissue Engineering
5.2.1. Collagen-Based Scaffolds
5.2.2. Fibrin-Based Scaffolds
5.3. Structure-Properties-Functionality Correlations in HVTE
6. Challenges and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peeters, F.E.C.M.; Meex, S.J.R.; Dweck, M.R.; Aikawa, E.; Crijns, H.J.G.M.; Schurgers, L.J.; Kietselaer, B.L.J.H. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J. 2018, 39, 2618–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlesnikar, T.; Delgado, V.; Bax, J.J. Imaging of valvular heart disease in heart failure. Card. Fail. Rev. 2018, 4, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Niazy, N.; Barth, M.; Selig, J.I.; Feichtner, S.; Shakiba, B.; Candan, A.; Albert, A.; Preuß, K.; Lichtenberg, A.; Akhyari, P. Degeneration of aortic valves in a bioreactor system with pulsatile flow. Biomedicines 2021, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Application of hydrogels in heart valve tissue engineering. J. Long-Term Eff. Med. Implants 2015, 25, 105–134. [Google Scholar] [CrossRef] [Green Version]
- Sellers, S.L.; Blanke, P.; Leipsic, J.A. Bioprosthetic heart valve degeneration and dysfunction: Focus on mechanisms and multidisciplinary imaging considerations. Radiol. Cardiothorac. Imaging 2019, 1, e190004. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, W.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of bioprosthetic heart valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Stassen, O.M.J.A.; Muylaert, D.E.P.; Bouten, C.V.C.; Hjortnaes, J. Current challenges in translating tissue-engineered heart valves. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- Schoen, F.J. Heart valve tissue engineering: Quo vadis? Curr. Opin. Biotechnol. 2011, 22, 698–705. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Bouten, C.V.C.; Smits, A.I.P.M.; Baaijens, F.P.T. Can We Grow Valves Inside the Heart? Perspective on Material-based In Situ Heart Valve Tissue Engineering. Front. Cardiovasc. Med. 2018, 5, 54. [Google Scholar] [CrossRef]
- Bouten, C.V.C.; Dankers, P.Y.W.; Driessen-Mol, A.; Pedron, S.; Brizard, A.M.A.; Baaijens, F.P.T. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Karagkiozaki, V.; Choli-Papadopoulou, T.; Mavromanolis, C.; Laskarakis, A.; Logothetidis, S. Development of biofunctionalized cellulose acetate nanoscaffols for heart valve tissue engineering. World J. Nano Sci. Eng. 2016, 6, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.M.; Drews, J.D.; Breuer, C.K. Tissue-engineered heart valves: A call for mechanistic studies. Tissue Eng. Part B Rev. 2018, 24, 240–253. [Google Scholar]
- Badria, A.F.; Koutsoukos, P.G.; Mavrilas, D. Decellularized tissue-engineered heart valves calcification: What do animal and clinical studies tell us? J. Mater. Sci. Mater. Med. 2020, 31, 132. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Vlierberghe, S.V.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue egineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Jana, S.; Tefft, B.J.; Spoon, D.B.; Simari, R.D. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014, 10, 2877–2893. [Google Scholar] [CrossRef]
- Urciuolo, A.; De Coppi, P. Decellularized tissue for muscle regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, S.D. Polymeric scaffolds in tissue engineering application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VeDepo, M.C.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of rapid prototyping techniques for tissue engineering scaffolds fabrication. In Characterization and Development of Biosystems and Biomaterials, 1st ed.; Öchsner, A.A., da Silva, L.F.M., Altenbach, H., Eds.; Springer: Berlin, Germany, 2013; Chapter 29; pp. 33–54. [Google Scholar]
- Kankala, R.K.; Xu, X.M.; Liu, C.G.; Chen, A.Z.; Wang, S.B. 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef] [Green Version]
- Buj-Corral, I.; Bagheri, A.; Petit-Rojo, O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials 2018, 11, 1532. [Google Scholar] [CrossRef]
- Farahani, R.; Dubé, M.; Therriault, D. Three-dimensional printing of multifunctional nanocomposites: Manufacturing techniques and applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.S.; Wang, S.B.; Chen, A.Z. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Weber, M.; de Torre, G.I.; Moreira, R.; Frese, J.; Oedekoven, C.; Alonso, M.; Cabello, R.C.J.; Jockenhoevel, S.; Mela, P. Multiple-step injection molding for fibrin-based tissue-engineered heart valves. Tissue Eng. Part C Methods 2015, 21, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.Y.; Duan, B.; Butcher, J.T. Current progress in tissue engineering of heart valves: Multiscale problems, multiscale solutions. Expert Opin. Biol. Ther. 2015, 15, 1155–1172. [Google Scholar] [CrossRef] [Green Version]
- Saleh, Q.; Moscona, J.; Le Jemtel, T. Valvular heart disease. In 3D Printing Applications in Cardiovascular Medicine, 1st ed.; Al’Aref, S.J., Mosadegh, B., Dunham, S., Min, J.K., Eds.; Elsevier: San Diego, CA, USA, 2018; Chapter 6; pp. 103–139. [Google Scholar]
- Qasim, M.; Haq, F.; Kang, M.H.; Kim, J.H. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int. J. Nanomed. 2019, 14, 1311–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng. Part C 2008, 14, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-based scaffolds in regenerative engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Doroftei, F.; Cazacu, G.; Cazacu, M. Morphological and surface aspects of cellulose-lignin hydrogels. Cell. Chem. Technol. 2013, 47, 377–386. [Google Scholar]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-based hydrogels for tissue engineering applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Li, Z.; Guan, J. Hydrogels for cardiac tissue engineering. Polymers 2011, 3, 740–761. [Google Scholar] [CrossRef] [Green Version]
- Hjortnaes, J.; Schoen, F.J. Application of hydrogels in heart valve tissue engineering. In Gels Handbook, Fundamentals, Properties and Applications, Applications of Hydrogels in Regenerative Medicine; Demirci, U., Khademhosseini, A., Eds.; World Scientific Publishing: Hackensack, NJ, USA, 2016; Volume 2, Chapter 12; pp. 363–383. [Google Scholar]
- Long, L.; Wu, C.; Hu, X.; Wang, Y. Biodegradable synthetic polymeric composite scaffold-based tissue engineered heart valve with minimally invasive transcatheter implantation. Polym. Adv. Technol. 2020, 31, 2422–2432. [Google Scholar] [CrossRef]
- Vajda, J.; Milojević, M.; Maver, U.; Vihar, B. Microvascular tissue engineering—A review. Biomedicines 2021, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Kluin, J.; Talacua, H.; Smits, A.I.P.M.; Emmert, M.Y.; Brugmans, M.C.P.; Fioretta, E.S.; Dijkman, P.E.; Söntjens, S.H.M.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Capulli, A.K.; Emmert, M.Y.; Pasqualini, F.S.; Kehl, D.; Caliskan, E.; Lind, J.U.; Sheehy, S.P.; Park, S.J.; Ahn, S.; Weber, B.; et al. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 2017, 133, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amore, A.; Luketich, S.K.; Raffa, G.M.; Olia, S.; Menallo, G.; Mazzola, A.; D’Accardi, F.; Grunberg, T.; Gu, X.; Pilato, M.; et al. Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. Biomaterials 2018, 150, 25–37. [Google Scholar] [CrossRef]
- Jana, S.; Bhagia, A.; Lerman, A. Optimization of polycaprolactone fibrous scaffold for heart valve tissue engineering. Biomed. Mater. 2019, 14, 065014. [Google Scholar] [CrossRef]
- Saidy, N.T.; Wolf, F.; Bas, O.; Keijdener, H.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Biologically inspired scaffolds for heart valve tissue engineering via melt electrowriting. Small 2019, 15, 1900873. [Google Scholar] [CrossRef]
- Chiono, V.; Mozetic, P.; Boffito, M.; Sartori, S.; Gioffredi, E.; Silvestri, A.; Rainer, A.; Giannitelli, S.M.; Trombetta, M.; Nurzynska, D.; et al. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus 2014, 4, 20130045. [Google Scholar] [CrossRef] [Green Version]
- Capulli, A.K.; MacQueen, L.A.; Sheehy, S.P.; Parker, K.K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev. 2016, 96, 83–102. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahman, Y. Tissue engineering and regenerative medicine. In Biomaterials Science and Technology: Fundamentals and Developments; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 10; pp. 235–258. [Google Scholar]
- Sultana, N. Mechanical and biological properties of scaffold materials. In Functional 3D Tissue Engineering Scaffolds, Materials, Technologies and Applications; Deng, Y., Kuiper, J., Eds.; Elsevier: Duxford, UK, 2018; Chapter 1; pp. 1–21. [Google Scholar]
- Chaudhuri, R.; Ramachandran, M.; Moharil, P.; Harumalani, M.; Jaiswal, A.K. Biomaterials and cells for cardiac tissue engineering: Current choices. Mater. Sci. Eng. Part C 2017, 79, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug. Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Engelmayr, G.; Auguste, D.T.; da Silva, F.L.; Karp, J.M.; Saigal, R.; Langer, R. Three-dimensional scaffolds. In Principles of Tissue Engineering, 3rd ed.; Lanza, R., Langer, R., Vacanti, J.P., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2011; Chapter 25; pp. 359–374. [Google Scholar]
- Oveissi, F.; Naficy, S.; Lee, A.; Winlaw, D.S.; Dehghani, F. Materials and manufacturing perspectives in engineering heart valves: A review. Mater. Today Bio 2020, 5, 100038. [Google Scholar] [CrossRef]
- How the Heart Valves Work, Edwards Lifesciences Corporation. Available online: https://yourheartvalve.com/heart-basics/heart-valves (accessed on 11 March 2022).
- Taghizadeh, B.; Ghavami, L.; Derakhshankhah, H.; Zangene, E.; Razmi, M.; Jaymand, M.; Zarrintaj, P.; Zarghami, N.; Jaafari, M.R.; Shahri, M.M.; et al. Biomaterials in valvular heart diseases. Front. Bioeng. Biotechnol. 2020, 8, 529244. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Dijkman, P.E.; Emmert, M.Y.; Hoerstrup, S.P. The future of heart valve replacement: Recent developments and translational challenges for heart valve tissue engineering. Tissue Eng. Regen. Med. 2018, 12, e323–e335. [Google Scholar] [CrossRef]
- Gott, V.L.; Alejo, D.E.; Cameron, D.E. Mechanical heart valves: 50 years of evolution. Ann. Thorac. Surg. 2003, 76, S2230–S2239. [Google Scholar] [CrossRef]
- Russo, M.; Taramasso, M.; Guidotti, A.; Pozzoli, A.; Nietilspach, F.; Von Segesser, L.; Maisano, F. The evolution of surgical valves. Cardiovasc. Med. 2017, 20, 285–292. [Google Scholar]
- The Valves, Division of Medicine and Science, National Museum of American History, Smithsonian Institution. Available online: https://americanhistory.si.edu/mending-broken-hearts/valves (accessed on 16 March 2022).
- Mahmood, F.; Matyal, R.; Mahmood, F.; Sheu, R.D.; BA, R.F.; Kamal, R.; Khabbaz, K.R. Intraoperative Echocardiographic Assessment of Prosthetic Valves: A Practical Approach. J. Cardiothorac. Vasc. Anesth. 2018, 32, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Schopka, S.; Schmid, T.; Schmid, C.; Lehle, K. Current strategies in cardiovascular biomaterial functionalization. Materials 2010, 3, 638–655. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.A.; Warnes, C.A. Anticoagulation during pregnancy in women with prosthetic valves: Evidence, guidelines and unanswered questions. Heart 2015, 101, 430–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoen, F.J. Mechanisms of function and disease of natural and replacement heart valves. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; Kumar, S.R.; Starnes, V.A. The Ross procedure: An excellent option in the right hands. Ann. Transl. Med. 2016, 4, 1–3. [Google Scholar] [CrossRef]
- Pasquali, S.K.; Shera, D.; Wernovsky, G.; Cohen, M.S.; Tabbutt, S.; Nicolson, S.; Spray, T.L.; Marino, B.S. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J. Thorac. Cardiovasc. Surg. 2007, 133, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Henaine, R.; Roubertie, F.; Vergnat, M.; Ninet, J. Valve replacement in children: A challenge for a whole life. Arch. Cardiovasc. Dis. 2012, 105, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Perri, G.; Polito, A.; Esposito, C.; Albanese, S.B.; Francalanci, P.; Pongiglione, G.; Carotti, A. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular out flow tract reconstruction in patients with congenital heart disease. Eur. J. Cardiothorac. Surg. 2012, 41, 1320–1325. [Google Scholar] [CrossRef] [Green Version]
- Avolio, E.; Caputo, M.; Madeddu, P. Stem cell therapy and tissue engineering for correction of congenital heart disease. Front. Cell Dev. Biol. 2015, 3, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Fioretta, E.D.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef]
- Dohmen, P.M.; Lembcke, A.; Holinski, S.; Pruss, A.; Konertz, W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann. Thorac. Surg. 2011, 92, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Cebotari, S.; Lichtenberg, A.; Tudorache, I.; Hilfiker, A.; Mertsching, H.; Leyh, R.; Breymann, T.; Kallenbach, K.; Maniuc, L.; Batrinac, A.; et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006, 114, I132–I137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, A.; Cebotari, S.; Tudorache, I.; Haverich, A.; Sarikouch, S. Heart valve engineering: Decellularized allograft matrices in clinical practice. Biomed. Tech. 2013, 58, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Lisy, M.; Kalender, G.; Schenke-Layland, K.; Brockbank, K.G.M.; Biermann, A.; Stock, U.A. Allograft heart valves: Current aspects and future applications. Biopreserv. Biobank. 2017, 15, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Cebotari, S.; Tudorache, I.; Ciubotaru, A.; Boethig, D.; Sarikouch, S.; Goerler, A.; Lichtenberg, A.; Cheptanaru, E.; Barnaciuc, S.; Cazacu, A.; et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: Early report. Circulation 2011, 124, S115–S123. [Google Scholar] [CrossRef] [Green Version]
- Ruzmetov, M.; Shah, J.J.; Geiss, D.M.; Fortuna, R.S. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: A single-institution comparison. J. Thorac. Cardiovasc. Surg. 2012, 143, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Pfitzner, R.; Barecka, D.; Pawlikowski, M.; Kopytek, M.; Rudnicka-Sosin, L.; Majewska, E.; Mazur, M.; Marcinkowska, Z. Influence of cryopreservation on structural, chemical, and immunoenzymatic properties of aortic valve allografts. Transplant. Proc. 2018, 50, 2195–2198. [Google Scholar] [CrossRef]
- Bonetti, A.; Marchini, M.; Ortolani, F. Ectopic mineralization in heart valves: New insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves. J. Thorac. Dis. 2019, 11, 2126–2143. [Google Scholar] [CrossRef] [PubMed]
- Delmo Walter, E.M.; de By, T.M.M.H.; Meyer, R.; Hetzer, R. The future of heart valve banking and of homografts: Perspective from the Deutsches Herzzentrum Berlin. HSR Proc. Intensive Care Cardiovasc. Anesth. 2012, 4, 97–108. [Google Scholar]
- Aikins, J.; Koomson, A.; Ladele, M.; Al-Nusair, L.; Ahmed, A.; Ashry, A.; Amer Harky, A. Anticoagulation and antiplatelet therapy in patients with prosthetic heart valves. J. Card. Surg. 2020, 35, 3521–3529. [Google Scholar] [CrossRef]
- Etnel, J.R.G.; Elmont, L.C.; Ertekin, E.; Mokhles, M.M.; Heuvelman, H.J.; Roos-Hesselink, J.W.; de Jong, P.L.; Helbing, W.A.; Bogers, A.J.J.C.; Takkenberg, J.J.M. Outcome after aortic valve replacement in children: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2016, 151, 143–152.e1-3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, K.H.; Murphy, R.; Devbhandari, M.; Venkateswaran, R. Aortic valve replacement: Is porcine or bovine valve better? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, G.; Brizard, C.; Karl, T.R.; Neethling, L. An evaluation of Admedus’tissue engineering process-treated (ADAPT) bovine pericardium patch (CardioCel) for the repair of cardiac and vascular defects. Expert Rev. Med. Devices 2015, 12, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, A.G.; Tolis, G.T., Jr. Surgical treatment of valvular heart disease: Overview of mechanical and tissue prostheses, advantages, disadvantages, and implications for clinical use. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Lee, W.; Cooper, D.K.C. Xenograft bioprosthetic heart valves: Past, present and future. Int. J. Surg. 2015, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.; Pfaff, M.; Schöttler, F.; Schmidt, V.; Lichtenberg, A.; Akhyari, P. Reproducible in vitro tissue culture model to study basic mechanisms of calcific aortic valve disease: Comparative analysis to valvular interstitials cells. Biomedicines 2021, 9, 474. [Google Scholar] [CrossRef]
- Butcher, J.T.; Mahler, G.J.; Hockaday, L.A. Aortic valve disease and treatment: The need for naturally engineered solutions. Adv. Drug Deliv. Rev. 2011, 63, 242–268. [Google Scholar] [CrossRef]
- Schmidt, D.; Stock, U.A.; Hoerstrup, S.P. Tissue engineering of heart valves using decellularized xenogeneic or polymeric starter matrices. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Dagenais, F.; Ruel, M.; Al-Atassi, T.; Dumont, E.; Mohammadi, S.; Bernier, M.; Pibarot, P.; Rodés-Cabau, J. Aortic stenosis and small aortic annulus. Circulation 2019, 139, 2685–2702. [Google Scholar] [CrossRef]
- Sharabiani, M.T.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Tometzki, A.J.P.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic valve replacement and the ross operation in children and young adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Nietlispach, F. The future of valves for percutaneous insertion. Eur. Heart J. 2014, 35, 1569–1574. [Google Scholar] [PubMed] [Green Version]
- Matsuzaki, Y.; Wiet, M.G.; Boe, B.A.; Shinoka, T. The real need for regenerative medicine in the future of congenital heart disease treatment. Biomedicines 2021, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Rosser, B.A.; Chan, C.; Hoschtitzky, A. Surgical Management of valvular heart disease in mucopolysaccharidoses: A review of literature. Biomedicines 2022, 10, 375. [Google Scholar] [CrossRef]
- Mirani, B.; Nejad, S.P.; Simmons, C.A. Recent progress toward clinical translation of tissue-engineered heart valves. Can. J. Cardiol. 2021, 37, 1064−1077. [Google Scholar] [CrossRef]
- Li, K.Y.C. Bioprosthetic heart valves: Upgrading a 50-year old technology. Front. Cardiovasc. Med. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Garcia, A.; Oliver-De-La-Cruz, J.; Mirabet, V.; Gandía, C.; Villagrasa, A.; Sodupe, E.; Escobedo-Lucea, C. Heart valve tissue engineering: How far is the bedside from the bench? Expert Rev. Mol. Med. 2015, 17, e16. [Google Scholar] [CrossRef] [Green Version]
- Bačáková, L.; Novotná, K.; Pařízek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29–S47. [Google Scholar] [CrossRef]
- Quintessenza, A.; Jacobs, J.P.; Chai, P.J.; Morell, V.O.; Lindberg, H. Polytetrafluoroethylene bicuspid pulmonary valve implantation: Experience with 126 patients. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 20–27. [Google Scholar] [CrossRef]
- Choi, K.H.; Sung, S.C.; Kim, H.; Lee, H.D.; Kim, G.; Ko, H. Late results of right ventricular outflow tract reconstruction with a bicuspid expanded polytetrafluoroethylene valved conduit. J. Card. Surg. 2018, 33, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough hydrophilic polyurethane-based hydrogels with mechanical properties similar to human soft tissues. J. Mater. Chem. B 2019, 7, 3512–3519. [Google Scholar] [CrossRef]
- Chiriac, A.I.; Pastor, F.I.J.; Popa, V.I.; Aflori, M.; Ciolacu, D. Changes of supramolecular cellulose structure and accessibility induced by the processive endoglucanase Cel9B from Paenibacillus barcinonensis. Cellulose 2014, 21, 203–219. [Google Scholar] [CrossRef]
- Kodigepalli, K.M.; Thatcher, K.; Toni, W.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and biomechanics of the heart valve extracellular matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, H.; Shoji, T.; Sugiura, T.; Fukunishi, T.; Miyamoto, S.; Breuer, C.K.; Shinoka, T. Current status of cardiovascular tissue engineering. Int. J. Clin. Ther. Diagn. 2015, S3:001, 1–10. [Google Scholar]
- Mendelson, K.; Schoen, F.J. Heart valve tissue engineering: Concepts, approaches, progress, and challenges. Ann. Biomed. Eng. 2006, 34, 1799–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the interface of biomaterials to design effective scaffolds. J. Funct. Biomater. 2018, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. Aortic heart valve tissue regeneration. In Tissue and Organ Regeneration, Advances in Micro- and Nanotechnology, 1st ed.; Zhang, L.G., Khademhosseini, A., Webster, T., Eds.; Jenny Stanford Publishing: Boca Raton, FL, USA, 2014; Chapter 19; pp. 645–694. [Google Scholar]
- Butcher, J.T.; Nerem, R.M. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J. Heart Valve Dis. 2004, 13, 478–486. [Google Scholar]
- Jana, S.; Tranquillo, R.T.; Lerman, A. Cells for tissue engineering of cardiac valves. J. Tissue Eng. Regen. Med. 2015, 10, 804–824. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.O.; Fiane, A.; Vaage, J. Valve interstitial cells: The key to understanding the pathophysiology of heart valve calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A.I.P.M. Biomaterial-driven in situ cardiovascular tissue engineering—A multi-disciplinary perspective. NPJ Regen. Med. 2017, 2, 18. [Google Scholar] [CrossRef]
- Moreira, R.; Velz, T.; Alves, N.; Gesche, V.N.; Malischewski, A.; Schmitz-Rode, T.; Frese, J.; Jockenhoevel, S.; Mela, P. Tissue-engineered heart valve with a tubular leaflet design for minimally invasive transcatheter implantation. Tissue Eng. Part C Methods 2015, 21, 530–540. [Google Scholar] [CrossRef]
- Sodian, R.; Schaefermeier, P.; Abegg-Zips, S.; Kuebler, W.M.; Shakibaei, M.; Daebritz, S.; Ziegelmueller, J.; Schmitz, C.; Reichart, B. Use of human umbilical cord blood−derived progenitor cells for tissue-engineered heart valves. Ann. Thorac. Surg. 2010, 89, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Meng, M.; Wu, J.; Zhang, J.; Hou, Z.; Gao, H.; Xu, H.; Liu, B.; Tang, W.; Jiang, L.; et al. Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell. Res. Ther. 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latifi, N.; Lecce, M.; Simmons, C.A. Porcine umbilical cord perivascular cells for preclinical testing of tissue engineered heart valves. Tissue Eng. Part. C Methods. 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Nejad, S.P.; Santerre, J.P.; Simmons, C. Engineering pulmonary valve tissue sheets from human umbilical cord perivascular cells and electrospun polyurethane. Struct. Heart. 2020, 4, 203. [Google Scholar] [CrossRef]
- Mikryukov, A.A.; Mazine, A.; Wei, B.; Yang, D.; Miao, Y.; Gu, M.; Keller, G.M. BMP10 signaling promotes the development of endocardial cells from human pluripotent stem cell-derived cardiovascular progenitors. Cell Stem Cell. 2021, 28, 96–111.e7. [Google Scholar] [CrossRef] [PubMed]
- Fiorettaa, E.S.; von Boehmera, L.; Mottaa, S.E.; Lintasa, V.; Hoerstrupa, S.P.; Emmert, M.Y. Cardiovascular tissue engineering: From basic science to clinical application. Exp. Gerontol. 2019, 117, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Grande-Allen, K.J. Which biological properties of heart valves are relevant to tissue engineering? Front. Cardiovasc. Med. 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motta, S.E.; Lintas, V.; Fioretta, E.S.; Hoerstrup, S.P.; Emmert, M.Y. Off-the-shelf tissue engineered heart valves for in situ regeneration: Current state, challenges and future directions. Expert Rev. Med. Devices 2018, 15, 35–45. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Schmitt, B.A.; Loerakker, S.; Sanders, B.; Spriestersbach, H.; Fioretta, E.S.; Bruder, L.; Brakmann, K.; Motta, S.E.; Lintas, V.; et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci. Transl. Med. 2018, 10, eaan4587. [Google Scholar] [CrossRef] [Green Version]
- Motta, S.E.; Fioretta, E.S.; Dijkman, P.E.; Lintas, V.; Behr, L.; Hoerstrup, S.P.; Emmert, M.Y. Development of an off-the-shelf tissue-engineered sinus valve for transcatheter pulmonary valve replacement: A proof-of-concept study. J. Cardiovasc. Transl. Res. 2018, 11, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Duan, B.; Qin, X.; Butcher, J.T. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta Biomater. 2017, 51, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101A, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.; Neusser, C.; Kruse, M.; Mulderrig, S.; Wolf, F.; Spillner, J.; Schmitz-Rode, T.; Jockenhoevel, S.; Mela, P. Tissue-engineered fibrin-based heart valve with bio-inspired textile reinforcement. Adv. Healthc. Mater. 2016, 5, 2113–2121. [Google Scholar] [CrossRef]
- Schmidt, D.; Mol, A.; Neuenschwander, S.; Breymann, C.; Gössi, M.; Zund, G.; Turina, M.; Hoerstrup, S.P. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur. J. Cardio-Thoracic. Surg. 2005, 27, 795–800. [Google Scholar] [CrossRef]
- Syedain, Z.; Reimer, J.; Schmidt, J.; Lahti, M.; Berry, J.; Bianco, R.; Tranquillo, R.T. 6-Month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials 2015, 73, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Lerman, A.; Simari, R.D. In vitro model of a fibrosa layer of a heart valve. ACS Appl. Mater. Interfaces 2015, 7, 20012–20020. [Google Scholar] [CrossRef]
- Puperi, D.S.; Kishan, A.; Touchet, T.; Punske, Z.; Wu, Y.; Cosgriff-Hernandez, E.; West, J.; Grande-Allen, J. Electrospun polyurethane and hydrogel composite scaffolds as biomechanical mimics for aortic valve tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 1546–1558. [Google Scholar] [CrossRef]
- Weber, M.; Heta, E.; Moreira, R.; Gesche, V.N.; Schermer, T.; Frese, J.; Jockenhoevel, S.; Mela, P. Tissue-engineered fibrin-based heart valve with a tubular leaflet design. Tissue Eng. Part. C Methods 2014, 20, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syedain, Z.H.; Meier, L.A.; Reimer, J.M.; Tranquillo, R.T. Tubular heart valves from decellularized engineered tissue. Ann. Biomed. Eng. 2013, 41, 2645–2654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Mol, A.; Breymann, C.; Achermann, J.; Odermatt, B.; Gössi, M.; Neuenschwander, S.; Prêtre, R.; Genoni, M.; Zund, G.; et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation 2006, 114 (Suppl. 1), I125–I131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.; Achermann, J.; Odermatt, B.; Breymann, C.; Mol, A.; Genoni, M.; Zund, G.; Hoerstrup, S.P. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 2007, 116 (Suppl. 11), I64–I70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.T.; Yalcin, H.C.; Marei, H.E. Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef]
- Flanagan, T.C.; Sachweh, J.S.; Frese, J.; Schnöring, H.; Gronloh, N.; Koch, S.; Tolba, R.H.; Schmitz-Rode, T.; Jockenhoevel, S. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng. Part A 2009, 15, 2965–2976. [Google Scholar] [CrossRef]
- Schmidt, D.; Dijkman, P.E.; Driessen-Mol, A.; Stenger, R.; Mariani, C.; Puolakka, A.; Rissanen, M.; Deichmann, T.; Odermatt, B.; Weber, B.; et al. Minimally-invasive implantation of living tissue engineered heart valves a comprehensive approach from autologous vascular cells to stem cells. J. Am. Coll. Cardiol. 2010, 56, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, F.W.H.; Perry, T.E.; Yu, Y.; Sherwood, M.C.; Rabkin, E.; Masuda, Y.; Garcia, G.A.; McLellan, D.L.; Engelmayr, G.C., Jr.; Sacks, M.S.; et al. From stem cells to viable autologous semilunar heart valve. Circulation 2005, 111, 2783–2791. [Google Scholar] [CrossRef]
- Gottlieb, D.; Kunal, T.; Emani, S.; Aikawa, E.; Brown, D.W.; Powell, A.J.; Nedder, A.; Engelmayr, G.C., Jr.; Melero-Martin, J.M.; Sacks, M.S.; et al. In vivo monitoring of function of autologous engineered pulmonary valve. J. Thorac. Cardiovasc. Surg. 2010, 139, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Syedain, Z.H.; Lahti, M.T.; Johnson, S.L.; Robinson, P.S.; Ruth, G.R.; Bianco, R.W.; Tranquillo, R.T. Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovasc. Eng. Technol. 2011, 2, 101–112. [Google Scholar] [CrossRef]
- Motta, S.E.; Lintas, V.; Fioretta, E.S.; Dijkman, P.E.; Putti, M.; Caliskan, E.; Biefer, H.R.C.; Lipiski, M.; Sauer, M.; Cesarovic, N.; et al. Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: Towards native-like transcatheter pulmonary valve replacements. NPJ Regen. Med. 2019, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Dijkman, P.E.; Scherman, J.; Sanders, B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Driessen-Mol, A.; Emmert, M.Y.; Dijkman, P.E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R.; et al. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: Long-term functionality and rapid in vivo remodeling in sheep. J. Am. Coll. Cardiol. 2014, 63, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.; Syedain, Z.; Haynie, B.; Lahti, M.; Berry, J.; Tranquillo, R. Implantation of a tissue-engineered tubular heart valve in growing lambs. Ann. Biomed. Eng. 2017, 45, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domalik-Pyzik, P.; Chłopek, J.; Pielichowska, K. Chitosan-based hydrogels preparation, properties and applications. In Polymers and Polymeric Composites: A Reference Series, Cellulose-Based Superabsorbent Hydrogels, 1st ed.; Mondal, M.I.H., Ed.; Springer International Publishing AG, Part of Springer Nature: New York, NY, USA, 2019; Chapter 19; pp. 1–23. [Google Scholar]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides: Classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Silvestri, A.; Boffito, M.; Sartori, S.; Ciardelli, G. Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci. 2013, 13, 984–1019. [Google Scholar] [CrossRef]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for tissue engineering: Current landscape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ahmed, S.A.; Tabish, M.; Hasnain, M.S. Natural polysaccharides in tissue engineering applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications, 1st ed.; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 23; pp. 531–548. [Google Scholar]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-based biomaterials in tissue engineering: A review. Tissue Eng. B 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Juenet, M.; Meddahi-Pellé, A.; Letourneur, D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr. Polym. 2015, 116, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Cuy, J.L.; Beckstead, B.L.; Brown, C.D.; Hoffman, A.S.; Giachelli, C.M. Adhesive protein interactions with chitosan: Consequences for valve endothelial cell growth on tissue-engineering materials. J. Biomed. Mater. Res. 2003, 67A, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Dong, N.; Shi, J.; Chen, S.; Guo, C.; Hu, P.; Qi, H. Fabrication of a novel hybrid heart valve leaflet for tissue engineering: An in vitro study. Artif. Organs 2009, 33, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Gallyamov, M.O.; Chaschin, I.S.; Khokhlova, M.A.; Grigorev, T.E.; Bakuleva, N.P.; Lyutova, I.G.; Kondratenko, J.E.; Badun, G.A.; Chernysheva, M.G.; Khokhlov, A.R. Collagen tissue treated with chitosan solutions in carbonic acid for improved biological prosthetic heart valves. Mater. Sci. Eng. C 2014, 37, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Shital, P.; Chen, R.; Owida, A.; Morsi, Y. Biomimetic electrospun gelatin-chitosan polyurethane for heart valve leaflets. J. Mech. Med. Biol. 2010, 10, 563–576. [Google Scholar] [CrossRef]
- Albanna, M.Z.; Bou-Akl, T.H.; Blowytsky, O.; Walters, H.L., III; Matthew, H.W.T. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Jahnavi, S.; Saravanan, U.; Arthi, N.; Bhuvaneshwar, G.S.; Kumary, T.V.; Rajan, S.; Verma, R.S. Biological and mechanical evaluation of a bio-hybrid scaffold for autologous valve tissue engineering. Mater. Sci. Eng. C 2017, 73, 59–71. [Google Scholar] [CrossRef]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Aubin, H.; Ahari, A.F.; Bae, H.; Nichol, J.W.; Khademhosseini, A. Surface-modified hyaluronic acid hydrogels to capture endothelial progenitor cells. Soft Matter 2010, 6, 5120–5126. [Google Scholar] [CrossRef] [Green Version]
- Prawel, D.A.; Dean, H.; Forleo, M.; Lewis, N.; Gangwish, J.; Popat, K.C.; Dasi, L.P.; James, S.P. Hemocompatibility and hemodynamics of novel hyaluronan-polyethylene materials for flexible heart valve leaflets. Cardiovasc. Eng. Technol. 2014, 5, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Simon-Walker, R. Hemocompatibility of Hyaluronan Enhanced Linear Low Density Polyethylene for Heart Valve Leaflet Applications. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2018. [Google Scholar]
- Heitkemper, M.; Hatoum, H.; Dasi, L.P. In vitro hemodynamic assessment of a novel polymeric transcatheter aortic valve. Mech. Behav. Biomed. Mater. 2019, 98, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kapetanovic, E.; Kang, K.H.; Butcher, J.T. Stiffness and adhesivity control aortic valve interstitial cell behavior within hyaluronic acid based hydrogels. Acta Biomater. 2013, 9, 7640–7650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, M.; Vrana, N.E.; Zorlutuna, P.; Sant, S.; Jung, S.; Masoumi, N.; Khavari-Nejad, R.A.; Javadi, G.; Khademhosseini, A. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J. Biomater. Appl. 2014, 29, 399–410. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J. Biomed. Mater. Res. A 2022, 110, 76–91. [Google Scholar] [CrossRef]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol—Bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef]
- Millon, L.E.; Guhados, G.; Wan, W. Anisotropic polyvinyl alcohol—Bacterial cellulose nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 444–452. [Google Scholar] [CrossRef]
- Mohammadi, H.; Boughner, D.; Millon, L.E.; Wan, W.K. Design and simulation of a poly(vinyl alcohol)—Bacterial cellulose nanocomposite mechanical aortic heart valve prosthesis. Proc. Inst. Mech. Eng. H J. Eng. Med. 2009, 223, 697–711. [Google Scholar] [CrossRef]
- Mohammadi, H. Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behavior. Proc. Inst. Mech. Eng. H J. Eng. Med. 2011, 225, 718–722. [Google Scholar] [CrossRef]
- Hu, Y.; Su, X.; Lei, Y.; Wang, Y. A novel anti—Calcification method for bioprosthetic heart valves using dopamine—Modified alginate. Polym. Bull. 2019, 76, 1423–1434. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Nachlas, A.L.Y.; Li, S.; Davis, M.E. Developing a clinically relevant tissue engineered heart valve—A review of current approaches. Adv. Healthc. Mater. 2017, 6, 1700918. [Google Scholar] [CrossRef] [PubMed]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The evolution of polystyrene as a cell culture material. Tissue Eng. Part B 2018, 24, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.B.; Kim, D.; Park, H.; Kim, D.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanna, M.Z.; Bou-Akl, T.H.; Walters, H.L., III; Matthew, H.W.T. Improving the mechanical properties of chitosan-based heart valve scaffolds using chitosan fibers. J. Mech. Behav. Biomed. Mater. 2012, 5, 171–180. [Google Scholar] [CrossRef]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Jahnavi, S.; Kumary, T.V.; Bhuvaneshwar, G.S.; Natarajan, T.S.; Verma, R.S. Engineering of a polymer layered bio-hybrid heart valve scaffold. Mater. Sci. Eng. C 2015, 51, 263–273. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004-13. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Ma, M.G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.M.A.; Abdel-Raouf, M.E.S. Cellulose-based superabsorbent hydrogels. In Polymers and Polymeric Composites: A Reference Series, Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing AG, Part of Springer Nature: New York, NY, USA, 2019; Chapter 8; pp. 1–23. [Google Scholar]
- del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Natural biomaterials. In Introduction to Biomaterials—Basic Theory with Engineering Applications, 1st ed.; Cambrigde University Press: Cambridge, UK, 2014; Chapter 8; pp. 198–232. [Google Scholar]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-based hydrogels in tissue engineering applications. Cellulose Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Backdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, J.; Liu, S.; Zhao, P.; Lu, G.; Chen, J. The preparation, characterization and evaluation of regenerated cellulose/collagen composite hydrogel films. Carbohydr. Polym. 2014, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Ullah, M.W.; Israr, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Three-dimensionally microporous and highly biocompatible bacterial cellulose-gelatin composite scaffolds for tissue engineering applications. RSC Adv. 2016, 6, 110840–110849. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Castanheira, E.M.S.; Soares, R.; Gama, M. Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2011, 98A, 554–566. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lai, J.T. Mechanical analysis of biocomposite materials from bacterial cellulose and hydroxyapatite. J. Med. Biol. Eng. 2013, 2, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Saska, S.; Teixeira, L.N.; de Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Jiang, Y.; Xv, X.; Liu, D.; Yang, Z.; Zhang, Q.; Shi, H.; Zhao, G.; Zhou, J. Preparation of cellulose nanofiber-reinforced gelatin hydrogel and optimization for 3D printing applications. Bioresources 2018, 13, 5909–5924. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers. Int. J. Biol. Macromol. 2019, 124, 411–417. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Bačáková, L.; Novotná, K.; Sopuch, T.; Havelka, P. Cell interaction with cellulose-based scaffolds for tissue engineering: A review. In Cellulose and Cellulose Composites, 1st ed.; Mondal, M.I.H., Ed.; Nova Science Publishers: New York, NY, USA, 2015; Chapter 13; pp. 341–375. [Google Scholar]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. Polysaccharides and their derivatives. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 4; pp. 67–89. [Google Scholar]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.Y.; Farrell, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.I.; Pant, H.R.; Nam, K.T.; Nirmala, R.; Oh, H.J.; Kim, I.; Kim, H.Y. Effect of adhesive on the morphology and mechanical properties of electrospun fibrous mat of cellulose acetate. Carbohydr. Res. 2011, 346, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate composition effects on a neural stem cell–seeded scaffold. Tissue Eng. Part C 2009, 15, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ab-Rahim, S.; Selvaratnam, L.; Raghavendran, H.R.B.; Kamarul, T. Chondrocyte-alginate constructs with or without TGF-b1 produces superior extracellular matrix expression than monolayer cultures. Mol. Cell. Biochem. 2013, 376, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 4, e201604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Guo, K.; Cho, C.C. Synthesis and characterization of novel biodegradable unsaturated poly(ester amide)/poly(ethylene glycol) diacrylate hydrogels. J. Polym. Sci. A Polym. Chem. 2005, 43, 3932–3944. [Google Scholar] [CrossRef]
- Yang, F.K.; Zhao, B. Adhesion properties of self-polymerized dopamine thin film. Open Surf. Sci. J. 2011, 3, 115–122. [Google Scholar] [CrossRef]
- Taylor, P.M.; Cass, A.E.G.; Yacoub, M.H. Extracellular matrix scaffolds for tissue engineering heart valves. Prog. Pediatr. Cardiol. 2006, 21, 219–225. [Google Scholar] [CrossRef]
- Lu, Q.; Ganesan, K.; Simionescu, D.T.; Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 2004, 25, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Iyer, R.; Soundararajan, A.; Dobkin, D.; Vesely, I. Collagen-based tissue engineering as applied to heart valves. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005. [Google Scholar]
- Dreger, S.A.; Thomas, P.; Sachlos, E.; Chester, A.H.; Czernuszka, J.T.; Taylor, P.M.; Yacoub, M.H. Potential for synthesis and degradation of extracellular matrix proteins by valve interstitial cells seeded onto collagen scaffolds. Tissue Eng. 2006, 12, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Bruyneel, A.; Clarke, K.; Carr, C.; Czernuszka, J. Collagen-based scaffolds for potential application of heart valve tissue engineering. J. Tissue Sci. Eng. 2012, S11, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ali, M.S.; Lacerda, C.M.R. A three-dimensional collagen-elastin scaffold for heart valve tissue engineering. Bioengineering 2018, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.-H.; Zhao, M.; Lin, Y.-R.; Tian, X.-D.; Wang, Y.-D.; Wang, Z.-X.; Wang, L.-X. Degradable chitosan-collagen composites seeded with cells as tissue engineered heart valves. Heart Lung Circ. 2017, 26, 94–100. [Google Scholar] [CrossRef]
- Nazir, R.; Bruyneel, A.; Carr, C.; Czernuszka, J. Collagen type I and hyaluronic acid based hybrid scaffolds for heart valve tissue engineering. Biopolymers 2019, 110, e23278. [Google Scholar] [CrossRef]
- Flanagan, T.C.; Cornelissen, C.; Koch, S.; Tschoeke, B.; Sadweh, J.S.; Schmitz-Rode, T.; Jockenhoevel, S. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 2007, 28, 3388–3397. [Google Scholar] [CrossRef]
- Kaminski, A.; Klopsch, C.; Mark, P.; Yerebakan, C.; Donndorf, P.; Gäbel, R.; Eisert, F.; Hasken, S.; Kreitz, S.; Glass, A.; et al. Autologous valve replacement—CD133+ stem cell-plus-fibrin composite-based sprayed cell seeding for intraoperative heart valve tissue engineering. Tissue Eng. Part C 2011, 17, 299–309. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Bradee, A.R.; Kren, S.; Taylor, D.A.; Tranquillo, R.T. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng. Part A 2013, 19, 759–769. [Google Scholar]
- Du, J.; Zhu, T.; Yu, H.; Zhu, J.; Sun, C.; Wang, J.; Chen, S.; Wanga, J.; Guo, X. Potential applications of three-dimensional structure of silk fibroin/poly(ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Appl. Surf. Sci. 2018, 447, 269–278. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H. Current progress in 3D bioprinting of tissue analogs. SLAS Technol. 2019, 24, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P.T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merryman, W.D.; Huang, H.Y.S.; Schoen, F.J.; Sacks, M.S. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J. Biomech. 2006, 39, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.J.; Hamaia, S.W.; Gullberg, D.; Malcor, J.D.; Farndale, R.W. Selectivity of the collagen-binding integrin inhibitors, TC-I-15 and obtustatin. Toxicol. Appl. Pharmacol. 2021, 428, 115669. [Google Scholar] [CrossRef]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W.T. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef]
- Waterhouse, A.; Wise, S.G.; Ng, M.K.C.; Weiss, A.S. Elastin as a nonthrombogenic biomaterial. Tissue Eng. Part B 2011, 17, 93–99. [Google Scholar] [CrossRef]
- Flanagan, T.C.; Wilkins, B.; Black, A.; Jockenhoevel, S.; Smith, T.J.; Pandit, A.S. A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials 2006, 27, 2233–2246. [Google Scholar] [CrossRef]
- Barsotti, M.C.; Felice, F.; Balbarini, A.; Di Stefano, R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 301–310. [Google Scholar] [CrossRef]
- Lai, V.K.; Lake, S.P.; Frey, C.R.; Tranquillo, R.T.; Barocas, V.H. Mechanical behavior of collagen-fibrin co-gels reflects transition from series to parallel interactions with increasing collagen content. J. Biomech. Eng. 2012, 134, 0110041. [Google Scholar] [CrossRef]
- de Torre, I.G.; Weber, M.; Quintanilla, L.; Alonso, M.; Jockenhoevel, S.; Cabello, J.C.R.; Mela, P. Hybrid elastin-like recombinamer-fibrin gels: Physical characterization and in vitro evaluation for cardiovascular tissue engineering applications. Biomater. Sci. 2016, 4, 1361–1370. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhu, T.; Wu, Y.; Zhou, X.; Yang, X.; Wang, J.; Fang, J.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Incorporation of amoxicillin-loaded organic montmorillonite into poly(ester-urethane) urea nanofibers as a functional tissue engineering scaffold. Colloids Surf. B 2017, 151, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Polymers and Polymeric Composites: A Reference Series, Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing AG, Part of Springer Nature: New York, NY, USA, 2019; Chapter 28; pp. 887–908. [Google Scholar]




















| Type of Scaffolds | Advantages | Disadvantage |
|---|---|---|
| Porous |
|
|
| Microsphere |
|
|
| Hydrogel |
|
|
| Fibrous |
|
|
| Types of Valves | Definition | Advantages | Disadvantages | |
|---|---|---|---|---|
| Mechanical valves |
|
|
| |
| Bioprosthetic valves | Autograft valves |
|
|
|
| Allograft valves (homograft) |
|
|
| |
| Xenograft valves (heterograft) |
|
|
| |
| Polymeric valves | Natural polymeric scaffolds |
|
|
|
| Synthetic polymeric scaffolds | ||||
| Composite polymeric scaffolds | ||||
| HVTE | Cell Sources | Study Conditions | Main Results | Ref. |
|---|---|---|---|---|
| In vitro | Human aortic valve interstitial cells (HAVICs) |
|
| [131] |
|
| [132] | ||
| HHghHuman aortic root smooth muscle cells (HAoSMCs) |
|
| [133] | |
| Human umbilical cord vein endothelial cells (HUVECs) |
|
| [120] | |
|
| [134] | ||
| Human umbilical cord blood cells (HUCBs) |
|
| [135] | |
|
| [121] | ||
| Human dermal fibroblasts (HDFn) and Ovine dermal fibroblast (ODF) |
|
| [136] | |
| Porcine aortic valve interstitial cells (PAVICs) |
|
| [137] | |
|
| [133] | ||
|
| [138] | ||
|
| [139] | ||
| Ovine umbilical vein endothelial cells (OUVECs) |
|
| [140] | |
| Ovine carotid arteries cells and Ovine umbilical arteries cells |
|
| [32] | |
| In situ | Ovine dermal fibroblast (oDF) |
|
| [141] |
|
| [136] |
| HVTE | Cell Sources | Study Conditions | Main Results | Ref. |
|---|---|---|---|---|
| In vitro | Human chorionic villous mesenchymal stem cells (CV-MSCs) |
|
| [142] |
| Human amniotic fluid cells (H-AFCs) |
|
| [143] | |
| Cardiac Stem Cells (eCSCs) from Adult Mouse Heart |
|
| [144] | |
| Porcine aortic valve interstitial cells (PAVICs) |
|
| [144] | |
| Ovine vascular-derived cells |
|
| [145] | |
|
| [146] | ||
| Ovine bone marrow-derived mesenchymal stromal cells (oBM-MSCs) |
|
| [147] | |
|
| [148] | ||
| In situ | Human dermal fibroblasts, neonatal (HDFn) |
|
| [149] |
|
| [150] | ||
| Human vascular-derived cells (vena saphena magna, VSM) |
|
| [151] | |
| Ovine vascular-derived cells |
|
| [145] | |
|
| [146] | ||
|
| [152] | ||
|
| [129] | ||
| Ovine bone marrow-derived mesenchymal stromal cells (oBM-MSCs) |
|
| [147] | |
|
| [148] | ||
| Ovine dermal fibroblast (oDF) |
|
| [141] | |
|
| [153] | ||
| Ovine peripheral vein-derived fibroblasts |
|
| [130] |
| Scaffold Types | Preparation Methods | Results | Ref. |
|---|---|---|---|
| Chitosan-Based Scaffolds | |||
| CH films | Casting method to form films; Adsorption of protein sol. on CH films (4 °C, overnight). | CH films: FI/SMCs is less spread and more elongated; CH/AP: modest VECs growth, altered elongated morphology, low spreading; CH/COL IV composites: enhanced VECs growth, superior cell morphology. | [164] |
| CH/AP | |||
| CH/COL composites | |||
| (bFGF-CH-P4HB)/DPAV hybrid scaffolds | Coating DPAV with bFGF-CH-P4HB by electrospinning technique (20 kV, room temp.) | bFGF-CH-P4HB fibers form membranes with uniform thickness, firmly attached on DPAV surface; bFGF has a positive effect on the MSCs proliferation. | [165] |
| CH/BP scaffolds | Immersion of BP tissues in CH/H2CO3 sol. (pH 3, 2 h, 30 MPa, room temp.) | CH/BP are less rigid and the risk factor of fatigue failureis reduced; Calcification and bacterial strains adhesion are attenuated; In vivo: no inflammatory reaction, after 4 months of implantation in rats. | [166] |
| CH-PU-GEL nanofibrous scaffolds | Electrospinning technique (16 to 20 kV, room temp.) | OCAs adhered preferentially on CH-GEL-PU, are flattened, spread across the surface and have cobblestone morphology; able to withstand shear-stresses ranging from 0.062 to 0.185 N/m2 for up to 3 h; | [167] |
| CH fibers with immobilized HEP | Extrusion method; HEP immobilization with EDC | Crosslinking degree influences fiber diameters, strength and stiffness; CH-HEP promotes VIC attachment and growth (cell viability ~ 95%, 10 days). | [168] |
| CH-PCL/DBP biohybrid scaffolds | Electrospinning technique (27–32 °C, 15 kV) | hVICs viability on CH-PCL/DBP (A&R) ~ 90%; Biohybrid (A) has better uniaxial mechanical properties and higher alignment of hVICs compared to a randomly electrospun sample (B). | [169] |
| Hyaluronic Acid-Based Scaffolds | |||
| Me-HA, Me-HA/PEG-DA hydrogels | Photopolymerization (UV light, 5 mW/cm2, 3 min, photoinitiator) | Degradation rate: Me-HA/PEG-DA—1 week; Me-HA—2 days; VICs remain viable following photopolymerization; high proliferation after exposure to LMW HA degradation products. | [170] |
| (Me-HA+CD34)/Me-Gel hydrogels | Photopolymerization (UV light, 180 s, 5.5 mW/cm2); CD34 immobilization by EDC/NHS. | Increasing CD34 conc. increases EPC attachment (25.3 ± 5.3 EPCs/mm2 at 10 μg/mL; 52.2 ± 5.0 EPCs/mm2 at 25 μg/mL); (Me-HA+CD34)/Me-Gel promoted cell elongationand higher spreading. | [171] |
| SilylHA-CTA/LLDPE IPNs | Silylation of HA-CTA; LLDPE films swollen in silylHA-CTA/xylene (50 °C/1 h). | HA/LLDPE exhibit lower contact angles and less blood clotting than LLDPE alone, which led to considerable thrombus formation; PHVs showed acceptable values for RF (4.77 ± 0.42%) and EOA (2.34 ± 0.5 cm2). | [172,173] |
| HA-LLDPE IPNs/CoCr-MP35N stent | Swelling process was used to obtain IPNs; fixing by PP sutures on the stent frame. | Hemodynamic parameters (EOA, RF, PI) have values comparable with those of commercial transcatheter valves; Turbulent flow tests show a decrease of RSS at each cardiac phase. | [174] |
| Me-HA/Me-Gel MOHA/Me-Gel hybridhydrogels | Molding technique and exposure to UV light (2 mW/cm2; 5 min) | Me-Gel stimulates VICs spread and migration from spheroids; Cell circularity was much lower in low stiffness hydrogels than in stiffer ones; VICs have a spindle-like morphology only in hydrogels with Me-Gel. | [175] |
| Me-HA/Me-Gel/PGS-PCL hybrid hydrogels | Immersion of electrospun PGS-PCL into hydrogel; Photocrosslinking (UV light, 45 s, 2.6 mW/cm2). | MVICs have an initial rounded shape and low spread; MVICs are predominantly spread over the surface of PGS-PCL fibers only; 21 days: MVICs spread is complete into hybrid hydrogels, with non-homogenous distribution at different depths. | [176] |
| Cellulose-Based Scaffolds | |||
| CA coatings for metallic valves | Electrospinning technique; Surface functionalization with RGD and YIGSRG | CA coatings promote cardiac cell growth on valve surface; CA ensures the control of endothelialization and reduction of thrombosis. | [12] |
| CNF/PU films nanocomposites | Film-stacking method; Compression molding | Prosthetic valves have good biological durability, fatigue resistance and hemodynamics properties; no failure is registered after accelerated fatigue tests, equivalent of 12-year cycles. | [177] |
| mNG composite hydrogels | Covalent conjugation of mNCC on Me-Gel backbone via NHS/EDC crosslinking | Encapsulated HADMSCs on mNG displayed phenotypic properties found within the heart valve spongiosa; lower expression of osteogenic genes indicates resistance toward calcification. | [178] |
| BC/PVA anisotropic nanocomposites | Physical crosslinking by freeze-thaw cycles (20 °C/−20 °C); molding technique | Mechanical properties are similar to valve leaflet tissues, in both principal directions; the composition and number of freeze-thaw cycles substantially influence the tissue properties. | [179,180] |
| Thermal processing; molding technique | Trileaflet mechanical heart valve mimics the non-linear mechanical properties and anisotropic behavior of the porcine heart valves. | [181,182] | |
| Alginate-Based Scaffolds | |||
| PEG-DA/Alg hydrogels | Simultaneous 3D printing/photocrosslink ingmethods | The scaffolds with 10% Alg allow PAVICs to grow along the conduits surface, but less on the root and leaflet interstitium; high cell viability: 91.3 ± 10.7% (day 1) and 100% (day 7 and 21). | [137] |
| Alg/GEL hydrogels | 3D bioprinting with mold extrusion technique | Printing accuracy: 84.3 ± 10.9%; Cell viability (7 days): 81.4 ± 3.4% (SMCs); 83.2 ± 4.0% (VICs); SMCs express α-SMA in stiff matrix; VICs express VIM in soft matrix. | [133] |
| Dop-Alg hydrogel coatings | Covalent bonding of Dop to Alg (EDC/NHS route); Crosslinking with GA | In vitro: only Dop-Alg determines a decrease in the Ca content: 2.919 ± 0.252 mg/L—day 3; 0.725 ± 0.012 mg/L—day 6; In vivo: the largest decrease in Ca content for Dop-Alg: 1.737 ± 0.124 mg/L—day 20; 0.675 ± 0.084 mg/L—day 30. | [183] |
| Scaffold Types | Preparation Methods | Results | Ref. |
|---|---|---|---|
| Collagen-Based Scaffolds | |||
| 3D-COL biological scaffolds | Decellularization by SDS extraction; crosslinking (EDC/NSH); enzymatic treatment to remove elastic fibers. | Mechanical properties of 3D-COL controlled by crosslinking degree; 3T3 cells adhere and proliferate on COL scaffolds and infiltrated to depth of about 20 mm after 7 days, and 40 mm after 28 days. | [227] |
| COL/NRASMC matrix | Collagen-cell suspension was cast into silicon rubber wells and cultured in an incubator. | Uniform tension, during COL compaction, increases the cell content, stimulates their metabolism and leads to stronger constructs; NRASMCs are metabolically active proved by the elastin inside and around the COL fibers, and the proteoglycans at their interface. | [228] |
| 3D COL disc scaffolds | Molding technique by rapid prototyping with 3D inkjet printer | VICs proliferate more on 1% w/v COL than 2% or 5%; VICs remodel the scaffold and synthesize new matrix (detection of remodeling enzymes, MMPs and ECM gene expression). | [229] |
| COL-EL; COL-C4S heterogenous scaffolds | Molding technique using PTFE molds, followed by freeze drying | Good cell proliferation on COL, due to natural cells binding via integrin receptors; C4S increase the cell metabolic activities; Low cell proliferation on EL, due to its non-integrinsignaling pathway. | [230] |
| COL-EL bilayer scaffolds | Solution casting into PTFE molds; freeze drying, repeated twice to obtain bilayer structure. | Bilayer scaffolds have anisotropic bending moduli similar to native valves CDCs prefer COL over EL when proliferating, resulting in asymmetrical cell distribution in the two different layers. | [210] |
| 3D COL-EL hydrogels scaffolds | COL-EL composition: 50% COL, 12% EL, 10% PBS, 28% equal parts of DMEM and FBS; pH 7.5; 37°C; 1 h. | 3D COL-EL scaffolds support cell attachment, proliferation and differentiation: after 7 days, VICs double their number and exhibited stable levels of integrin β1 and F-actin expression; VECs have a very good proliferation, but the integrin β1 expression remained low. | [231] |
| 3D COL-CH composites scaffolds | COL:CH (7:1, w/w) The composites were seeded with 3 types of cells: SMCs, FIs and ECs. | 3D COL-CH have good cells adhesion and support ECs differentiation; SMCs group—large number of SMCs with dense disordered arrangement; SMC+EC group: large number of scattered ECs with long shuttle shape. | [232] |
| COL-HA hybrid scaffolds | Crosslinking by EDC/NHS route. | Structure similar to fibrosa layer of the valve leaflets; CDCs attachment not affected by the pore size and stiffness. | [233] |
| Fibrin-Based Scaffolds | |||
| Autologous fibrin-based heart valve scaffolds | Molding technique; In vitro: bioreactor conditioning; In vivo: implantation in sheep pulmonary trunk (3 months). | In vitro: well-organized structure of “conditioned samples”, aligned OCAs in leaflets; cellular detachment, possible cells death in “control samples”; In vivo: fibrin scaffolds completely resorbed and replaced by ECM proteins; significant tissue development and cell distribution. | [145,234] |
| (SC-F) composites biological valves | Coating DPPV with stem cells-fibrin complex | Static condition: 1st day—homogenous distribution of SC; 16th day—cell colony formation in SC-F compared to control (no cell clusters); Dynamic conditions: starting with the 4th day, floating composite clots at the inner surface of the valve and leaflets are observed. | [235] |
| Fibrin-based tubular heart valves | The tube mounted on a frame with three struts which, upon back-pressure, cause the tube to collapse into three coating “leaflets”. | In vitro: excellent performance under hydrodynamic conditions, minimal RF (approx. 5%), excellent values for TGV and EOA; In vivo (sheep, 2 months): substantial recellularization and no significant change in diameter or mechanical properties. | [236] |
| Tubular construct sutured at the root circumferential line and at three single points of sinotubular junction. | Advantage of one-piece construct manufacturing method without glue; In vivo (sheep, 3 months): no thrombus formation, calcification or stenosis; formation of ECs confluent monolayer on the valve surface. | [140] | |
| Fibrin-based tube-in-stent heart valves | Fibrin gel and HUVCs molded as tube-in-stent form and sewn into a self-expandable nitinol stent. | Homogeneous cells distribution throughout the valve; The simulation of the catheter-based delivery (the valves crimping for 20 min) does not influence the valve mechanical properties or functionality. | [120] |
| F-ELR biomimetic heart valves | Multi-step injection molding: the valve wall obtained from F gel and the leaflets from F-ELR gel. | Good structure cohesion and functionality (opening/closing cycles); Different cell type localization: the vessel-derived α-SMA negative (leaflets) and α-SMA positive cells (valve wall). | [32] |
| F/PLDL-PLGA anisotropic composites BioTexValve | Molding of PLDL multifilaments and electrospun PLGA fibers incorporated within fibrin gel. | Anisotropic Young’s moduli comparable with the native aortic leaflets; The valve withstands aortic flow/pressure conditions in flow-loop system; Homogeneous distribution of α-SMA, aligned with the longitudinal direction of the wall and leaflets. | [134] |
| SF/LDI-PEUU nanofibrous scaffolds | SF and LDI-PEUU prepared by electrospinning process. | Smooth and porous 3D structure of SF/LDI-PEUU scaffolds with randomly oriented fibers; Good blood compatibility (hemolysis rate <5%); HUVECs have spindle-shaped morphology and good spread. | [237] |
| Composition | Pore Size (μm) | D4/D1 | D7/D1 |
|---|---|---|---|
| 100% COL | 147.6 ± 38.4 | 4.63 ± 0.32 | 9.39 ± 0.86 |
| 50% COL + 50% EL | 179.9 ± 35.8 | 4.70 ± 0.39 | 5.53 ± 0.69 |
| 20% COL + 80% EL | 187.6 ± 36.5 | 3.68 ± 0.30 | 5.24 ± 0.36 |
| 90% COL + 10% C4S | 115.6 ± 27.6 | 3.64 ± 0.15 | 10.29 ± 0.63 |
| 50% COL + 50% C4S | 116.8 ± 17.8 | 3.10 ± 0.19 | 9.15 ± 0.95 |
| Scaffold type | Specific Structural Properties | Functionality as TEHV Scaffold | Ref. |
|---|---|---|---|
| Films/membranes |
|
| [164,165] |
| Extruded fibrous scaffolds |
|
| [168] |
| Electrospun nanofibrous scaffolds |
|
| [167,169,237] |
| Hydrogels/Hybrid hydrogels |
|
| [170,171,175,176] |
| Nanocomposites/Anisotropic nanocomposites |
|
| [178,181,182] |
| IPNs hydrogels |
|
| [172,173] |
| Polymer-bioprosthetic valves composites |
|
| [166,174,235] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051095
Ciolacu DE, Nicu R, Ciolacu F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines. 2022; 10(5):1095. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051095
Chicago/Turabian StyleCiolacu, Diana Elena, Raluca Nicu, and Florin Ciolacu. 2022. "Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges" Biomedicines 10, no. 5: 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines10051095