Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance
Abstract
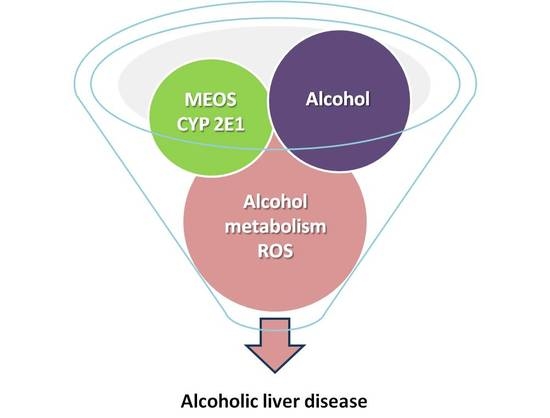
:1. Introduction
2. Literature Search and Data Review
3. Alcohol and Acetaldehyde Metabolism
3.1. Alcohol Metabolism
3.1.1. Alcohol Dehydrogenase
3.1.2. Microsomal Ethanol-Oxidizing System
3.2. Overall Alcohol Metabolism
3.3. Acetaldehyde Dehydrogenase
4. Reactive Oxygen Species
5. Gut–Liver Axis
6. Hepatic Active Mediators and Signaling Pathways in ALD
6.1. Alcoholic Fatty Liver
6.2. Alcoholic Steatohepatitis and Alcoholic Hepatitis
6.3. Alcoholic Fibrosis and Cirrhosis
6.4. Alcoholic Hepatocellular Carcinoma
7. Risk Factors
7.1. Amount of Consumed Alcohol
7.2. Gender
7.3. Genetic Predisposition
7.4. Alcohol-Unrelated Liver Disease
8. Conclusions
Funding
Conflicts of Interest
References
- WHO. Global Status Report on Alcohol and Health; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/substance_abuse/publications/alcohol_2011/en/ (accessed on 20 August 2019).
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Neuman, M.G.; French, S.W.; French, B.A.; Seitz, H.K.; Cohen, L.B.; Mueller, S.; Osna, N.A.; Kharbanda, K.K.; Seth, D.; Bautista, A.; et al. Alcoholic and non-alcoholic steatohepatitis. Exp. Mol. Pathol. 2014, 97, 492–510. [Google Scholar] [CrossRef] [Green Version]
- Bergheim, I.; McClain, C.; Arteel, G.E. Treatment of alcoholic liver disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic steatohepatitis (ASH) and alcoholic hepatitis (AH): Cascade of events, clinical aspects, and pharmacotherapy options. Expert Opin. Pharm. 2018, 19, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Liver Injury by Carbon Tetrachloride Intoxication in 16 Patients Treated with Forced Ventilation to Accelerate Toxin Removal via the Lungs: A Clinical Report. Toxics 2018, 6, 25. [Google Scholar] [CrossRef]
- Teschke, R. Intoxications by aliphatic halogenated hydrocarbons: Hepatotoxic risks for patients and clinical issues including role of CO2-induced hyperventilation as therapy option. J. Clin Exp Tox 2018, 2, 25–29. [Google Scholar]
- Teschke, R. Aliphatic halogenated hydrocarbons: Liver injury in 60 patients. J. Clin. Transl. Hepatol. 2018, 6, 1–12. [Google Scholar]
- Teschke, R.; Zhu, Y. Opinion: Intestinal microbiome, endotoxins, cytochrome P450 2E1, and the gut-liver axis in alcoholic liver disease. EC Gastroenterology Dig. Syst. 2019, 6, 66–75. [Google Scholar]
- Teschke, R. Idiosyncratic DILI: Analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front. Pharm. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Niu, M.; Chen, J.; Zou, Z.S.; Ma, Z.J.; Liu, S.H.; Wang, R.L.; He, T.T.; Song, H.B.; Pu, S.B.; et al. Comparison between Chinese herbal medicine and western medicine-induced liver injury of 1985 patients. J. Gastroenterol Hepatol. 2016, 31, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Teschke, R. Traditional Chinese medicine (TCM) and herb induced liver injury: Comparison with drug induced liver injury. J. Clin. Transl. Hepatol. 2018, 6, 57–68. [Google Scholar] [PubMed]
- Lieber, C.S.; DeCarli, L.M. The Feeding of Alcohol in Liquid Diets: Two Decades of Applications and 1982 Update. Alcohol. Clin. Exp. Res. 1982, 6, 523–531. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-De Carli diet-a flagship model for experimental alcoholic liver disease (ALD). Alcohol Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef]
- Gao, B.; Xu, M.-J.; Bertola, A.; Wang, H.; Zhou, Z.; Liangpunsakul, S. Animal Models of Alcoholic Liver Disease: Pathogenesis and Clinical Relevance. Gene Expr. 2017, 17, 173–186. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; Del Moral, M.G.; Martínez-Naves, E.; A Nevzorova, Y.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef]
- Lieber, C. Mechanism of ethanol induced hepatic injury. Pharm. Ther. 1990, 46, 1–41. [Google Scholar] [CrossRef]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef] [Green Version]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomed. 2018, 6, 106. [Google Scholar] [CrossRef]
- Logan, B.K.; Jones, A.W. Endogenous Ethanol ‘Auto-Brewery Syndrome’ as a Drunk-Driving Defence Challenge. Med. Sci. Law 2000, 40, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Cordell, B.J.; Kanodia, A.; Miller, G.K. Case–Control Research Study of Auto-Brewery Syndrome. Glob. Adv. Heal. Med. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Teschke, R. Microsomal ethanol-oxidizing system (MEOS): Success over 50 years and an encouraging future. Alcohol Clin. Exp. Res. 2019, 43, 386–400. [Google Scholar] [CrossRef]
- Seitz, H.K.; Egerer, G.; Simanowski, U.A.; Waldherr, R.; Eckey, R.; Agarwal, D.P.; Goedde, W.H.; von Wartburg, J.P. Human gastric alcohol dehydrogenase activity: Effect of age, gender and alcoholism. Gut 1993, 34, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Caballeria, J.; Frezza, M.; Hernández-Muñoz, R.; DiPadova, C.; Korsten, M.; Baraona, E.; Lieber, C. Gastric origin of the first-pass metabolism of ethanol in humans: Effect of gastrectomy. Gastroenterol. 1989, 97, 1205–1209. [Google Scholar] [CrossRef]
- Frezza, M.; di Padova, C.; Pozzato, G.; Terpin, M.; Baraona, E.; Lieber, C.S. High blood alcohol levels in women-Role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N. Engl. J. Med. 1990, 322, 95–99. [Google Scholar] [CrossRef]
- Egerer, G.; Simanowski, U.A.; Schmier, M.; Chang, G.; Bogusz, M.; Seitz, H.K. Effect of age on the first pass metabolism of ethanol in man. In Biomedical and Social Aspects of Alcohol and Alcoholism: Proceedings of the Fourth Congress of the International Society for Biomedical Research on Alcoholism (ISBRA); Excerpta Medica: Amsterdam, The Netherlands; New York, NY, USA, 1988; pp. 115–118. [Google Scholar]
- Salaspuro, M. Epidemiological aspects of alcohol and alcoholic liver disease, ethanol metabolism, and pathogenesis of alcoholic liver injury. Oxf. Textb. Clin. Hepatol. 1991, 2, 791–810. [Google Scholar]
- Pizon, A.F.; Becker, C.E.; Bikin, D. The clinical variations in ethanol toxicokinetics. Toxicol Rev. 2007, 3, 63–72. [Google Scholar]
- Parker, R.; Kim, S.J.; Gao, B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M.; Matsuzaki, S.; Ohnishi, K.; Teschke, R. The microsomal ethanol oxidizing system (MEOS). Methods Enzym. 1978, 52, 355–368. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [CrossRef]
- Teschke, R.; Matsuzaki, S.; Ohnishi, K.; Hasumura, Y.; Lieber, C.S. Metabolism of alcohol at high concentrations: Role and biochemical nature of the hepatic microsomal ethanol oxidizing system. Adv. Exp. Med. Biol. 1977, 85, 257–280. [Google Scholar]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Seitz, H.K.; Korsten, M.A.; Lieber, C.S. Ethanol oxidation by intestinal microsomes: Increased activity after chronic ethanol administration. Life Sci. 1979, 25, 1443–1448. [Google Scholar] [CrossRef]
- Pronko, P.; Bardina, L.; Satanovskaya, V.; Kuzmich, A.; Zimatkin, S. Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol 2002, 37, 229–235. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bösche, P.; Czygan, P.; Veith, S.; Kommerell, B. Microsomal ethanol oxidation in the colonic mucosa of the rat: Effect of chronic ethanol ingestion. N.S. Arch. Pharm. 1982, 320, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Wang, X.D. The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem. 2013, 67, 131–143. [Google Scholar]
- Shimizu, M.; Lasker, J.M.; Tsutsumi, M.; Lieber, C.S. Immunohistochemical localization of ethanol-inducible P-450IIE1 in the rat alimentary tract. Gastroenterology 1990, 99, 1044–1053. [Google Scholar] [CrossRef]
- Bergheim, I.; Bode, C.; Parlesak, A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clin Pharm. 2005, 5, 4. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Ethanol oxidation by hepatic microsomes: Adaptive increase after ethanol feeding. Science 1968, 162, 917–918. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J. Biol. Chem. 1970, 245, 2505–2512. [Google Scholar]
- Lieber, C.S.; DeCarli, L.M. Reduced nicotinamide-adenine dinucleotide phosphate oxidase: Enhanced by ethanol consumption. Science 1970, 170, 78–80. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Joly, J.G.; Ishii, H.; Lieber, C.S. Microsomal ethanol-oxidizing system (MEOS): Purification and properties of a rat liver system free of catalase and alcohol dehydrogenase. Biochem. Biophys. Res. Commun. 1972, 49, 1187–1193. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. NADPH-dependent oxidation of methanol, ethanol, propanol, and butanol by hepatic microsomes. Biochem. Biophys. Res. Commun. 1974, 60, 851–857. [Google Scholar] [CrossRef]
- Teschke, R.; Ohnishi, K.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal ethanol oxidizing system: Isolation and reconstitution. Microsomes Drug Oxid. 1977, 103–110. [Google Scholar]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal alcohol-oxidizing system: Affinity for methanol, ethanol, propanol and butanol. J. Biol. Chem. 1975, 250, 7397–7404. [Google Scholar]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal ethanol oxidizing system: Solubilization, isolation and characterization. Arch. Biochem. Biophys. 1974, 163, 404–415. [Google Scholar] [CrossRef]
- Ohnishi, K.; Lieber, C.S. Reconstitution of the microsomal ethanol-oxidizing system. Qualitative and quantitative changes of cytochrome P-450 after chronic ethanol consumption. J. Biol. Chem. 1977, 252, 7124–7131. [Google Scholar]
- Miwa, G.T.; Lewin, W.; Thomas, P.E.; Lu, A.Y. The direct oxidation of ethanol by a catalase- free and alcohol dehydrogenase-free reconstituted system containing cytochrome P-450. Arch. Biochem. Biophys. 1978, 187, 464–475. [Google Scholar] [CrossRef]
- Damgaard, S.E. The D (V/K) isotope effect of the cytochrome P-450-mediated oxidation of ethanol and its biological applications. Eur. J. Biochem. 1982, 125, 593–603. [Google Scholar] [CrossRef]
- Udoh, U.S.; Valcin, J.A.; Gamble, K.L.; Bailey, S.M. The molecular circadian clock and alcohol-induced liver injury. Biomolecules 2015, 5, 2504–2537. [Google Scholar] [CrossRef]
- Sturtevant, R.P.; Garber, S.L. Circadian rhythms of alcohol dehydrogenase and MEOS in the rat. Proc. Soc. Exp. Biol. Med. USA 1984, 175, 299–303. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. The role of the hepatic microsomal ethanol-oxidizing system (MEOS) for ethanol metabolism in vivo. J. Pharm. Exp. Ther. 1972, 181, 279–287. [Google Scholar]
- Hasumura, Y.; Teschke, R.; Lieber, C.S. Acetaldehyde oxidation by hepatic mitochondria: Its decrease after chronic ethanol consumption. Science 1975, 189, 727–729. [Google Scholar] [CrossRef]
- Hasumura, Y.; Teschke, R.; Lieber, C.S. Characteristics of acetaldehyde oxidation in rat liver mitochondria. J. Biol. Chem. 1976, 251, 4908–4913. [Google Scholar] [PubMed]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol Rev 1997, 77, 517–544. [Google Scholar] [CrossRef]
- Tanaka, E.; Terada, M.; Misawa, S. Cytochrome P450 2E1: Its clinical and toxicological role. Clin. Pharm. Ther 2000, 25, 165–175. [Google Scholar] [CrossRef]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 47, 17756. [Google Scholar] [CrossRef]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatology 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Fukui, H.; Brauner, B.; Bode, J.C.; Bode, C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: Reevaluation with an improved chromogenic assay. J. Hepatology 1991, 12, 162–169. [Google Scholar] [CrossRef]
- Purohita, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a Symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.C.; Bode, C.; Heidelbach, R.; Dürr, H.K.; Martini, G.A. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology 1984, 31, 30–34. [Google Scholar]
- Schäfer, C.; Parlesak, A.; Schütt, C.; Bode, J.C.; Bode, C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcoholism 2002, 37, 81–86. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Banerjee, A.; Jang, S.; Yoo, S.H.; Yun, J.W.; Gonzalez, F.J.; Keshavarzian, A.; Song, B.J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013, 65, 1238–1245. [Google Scholar] [CrossRef]
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Gut-liver and sterile signals in the development of alcoholic liver disease. Alcohol Alcoholism 2017, 52, 414–424. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.; Zhang, C.L.; Xiao, M.; Yang, R.; Xie, K.Q. Critical roles of Kupffer cells in the pathogenesis of alcoholic liver disease: From basic science to clinical trials. Front. Immunol. 2016, 7, 538. [Google Scholar] [CrossRef]
- Suh, Y.G.; Jeong, W.I. Hepatic stellate cells and innate immunity in alcoholic liver disease. World J. Gastroenterol. 2011, 17, 2543–2551. [Google Scholar] [CrossRef]
- Reeves, H.L.; Burt, A.D.; Wood, S.; Day, C.P. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J. Hepatol. 1996, 25, 677–683. [Google Scholar] [CrossRef]
- De Leve, L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 2015, 61, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–844. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Tsukamoto, H.; Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001, 15, 1335–1349. [Google Scholar] [CrossRef] [Green Version]
- Mihm, S. Danger-associated molecular patterns (DAMPs): Molecular triggers for sterile inflammation in the liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Mehal, W.Z. Sterile inflammation in the liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent insights into the role of immune cells in alcoholic liver disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Savolainen, E.R.; Leo, M.A.; Timpl, R.; Lieber, C.S. Acetaldehyde and lactate stimulate collagen synthesis of cultured baboon liver myofibroblasts. Gastroenterology 1984, 87, 777–787. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 8, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Sidharthan, S.; Kottilil, S. Mechanisms of alcohol-induced hepatocellular carcinoma. Hepatol. Int. 2014, 8, 452–457. [Google Scholar] [CrossRef]
- Lelbach, W.K. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann. N.Y. Acad. Sci. 1975, 252, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Péquinot, G.; Tuyns, A.J.; Berta, J.L. Ascitic cirrhosis in relation to alcohol consumption. Int. J. Epidemiol. 1978, 7, 113–120. [Google Scholar] [CrossRef]
- Tuyns, A.J.; Péquinot, G. Greater risk of ascetic cirrhosis in females in relation to alcohol consumption. Int. J. Epidemiol. 1984, 13, 53–57. [Google Scholar] [CrossRef]
- Becker, U.; Deis, A.; Sorensen, T.I.A.; Gronbaeck, M.; Borch-Johnsen, K.; Müller, C.F.; Schnohr, P.; Jensen, G. Prediction of risk of liver disease by alcohol intake, sex, and age: A prospective population study. Hepatology 1996, 23, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Hampe, J. Genetic determinants of alcoholic liver disease. Gut 2012, 61, 150–159. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teschke, R. Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance. Biomedicines 2019, 7, 68. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030068
Teschke R. Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance. Biomedicines. 2019; 7(3):68. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030068
Chicago/Turabian StyleTeschke, Rolf. 2019. "Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance" Biomedicines 7, no. 3: 68. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines7030068