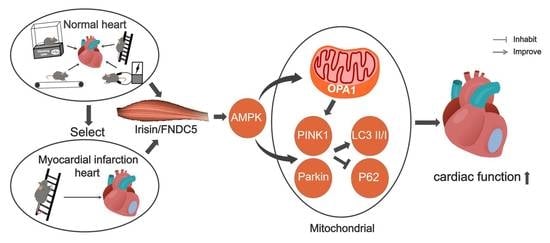
Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Exercise Protocol
2.2. Cell Culture
2.3. Echocardiographic Measurements
2.4. Blood Collection and Biochemical Index Measurement
2.5. Extraction and Detection of Mitochondrial Protein
2.6. RT-qPCR
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Different Types of Exercise and Skeletal Muscle Electrical Stimulation Intervention Enhanced Myocardial Irisin/FNDC5 Expression and Cardiac Function
3.2. Different Types of Exercise and Skeletal Muscle Electrical Stimulation Intervention Activated the Myocardial PINK1/Parkin Pathway and Enhanced Antioxidant Function
3.3. Different Types of Exercise and Skeletal Muscle Electrical Stimulation Intervention Activated the Mitochondrial PINK1/Parkin Pathway and Enhanced Antioxidant Function
3.4. Effect of Different Types of Exercise and Skeletal Muscle Electrical Stimulation Intervention on Myocardium L-OPA1/S-OPA1 Ratio and Its Correlation with Irisin/FNDC5
3.5. AICAR Intervention Enhanced H9C2 Cells Irisin/FNDC5 Expression, Increased Mitophagy and Antioxidant Function
3.6. Resistance Exercise Enhanced MI Myocardial Irisin/FNDC5 Expression, Promoted Mitophagy, Enhanced Antioxidative Capability and Alleviated the Levels of Oxidative Stress
3.7. Resistance Exercise Improved MI Mice Cardiac Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AICAR | 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide |
| AMPK | Adenosine monophosphate (AMP)-activated protein kinase |
| ANOVA | Analysis of variance |
| EF | Ejection fraction |
| FNDC5 | Fibronectin type III domain protein 5 |
| FS | Fractional shortening |
| LAD | Left anterior descending coronary artery |
| LC3 | Microtubule-associated protein light chain 3 |
| LVIDd | Left ventricle internal dimension diastole |
| LVIDs | Left ventricle internal dimension systole |
| MI | Myocardial infarction |
| MDA | Malondialdehyde |
| OPA1 | Optic atrophy protein-1 |
| P62 | SQSTM1/p62 |
| Parkin | E3 ubiquitin ligase parkin |
| PINK1 | PTEN-induced kinase 1 |
| RIPA | Radio-immunoprecipitation assay |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel |
| SOD2 | Superoxide dismutase 2 |
| T-SOD | Total Superoxide dismutase |
| WT | Wildtype |
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Sayer, G.; Annamalai, S.; Kapur, N.K.; Burkhoff, D. Mechanical unloading in heart failure. J. Am. Coll. Cardiol. 2018, 72, 569–580. [Google Scholar] [CrossRef]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef]
- Song, W.; Liang, Q.; Cai, M.; Tian, Z. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J. Cell. Mol. Med. 2020, 24, 12970–12979. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011, 162, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.B.; Gottlieb, R.A. Recycle or die: The role of autophagy in cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.-I.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1360–1371. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.S.; Baniyas, M.M.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.T.; Zhang, S.; Dubielecka, P.M.; Du, J.; Yano, N.; Chin, Y.E.; Zhuang, S.; Qin, G.; Zhao, T.C. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell. Physiol. 2017, 232, 3775–3785. [Google Scholar] [CrossRef]
- Kaludercic, N.; Maiuri, M.C.; Kaushik, S.; Fernández, Á.F.; de Bruijn, J.; Castoldi, F.; Chen, Y.; Ito, J.; Mukai, R.; Murakawa, T.; et al. Comprehensive autophagy evaluation in cardiac disease models. Cardiovasc. Res. 2020, 116, 483–504. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic, D.; Chau, K.; Schapira, A.H.V.; Gegg, M.E. Mitochondrial and lysosomal biogenesis are activated following PINK 1/parkin-mediated mitophagy. J. Neurochem. 2016, 136, 388–402. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Torrella, J.R.; Oliveira, P.J.; Magalhães, J.; Ascensão, A. Exercise and doxorubicin treatment modulate cardiac mitochondrial quality control signaling. Cardiovasc. Toxicol. 2018, 18, 43–55. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Rahmati-Ahmadabad, S. Irisin interaction with adipose tissue secretions by exercise training and flaxseed oil supplement. Lipids Health Dis. 2019, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Sonobe, T.; Tsuchimochi, H.; Schwenke, D.O.; Pearson, J.T.; Shirai, M. Treadmill running improves hindlimb arteriolar endothelial function in type 1 diabetic mice as visualized by X-ray microangiography. Cardiovasc. Diabetol. 2015, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Hashimoto, T.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Lan, Q.; Chen, Y.; Chan, Y.W.J.; Mahady, G.; Lee, S.M.-Y. Low-Magnitude High-Frequency Vibration Decreases Body Weight Gain and Increases Muscle Strength by Enhancing the p38 and AMPK Pathways in db/db Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 979–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Hu, L.; Cheng, J.; Klein, J.D.; Hassounah, F.; Cai, H.; Li, M.; Wang, H.; Wang, X.H. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J. Appl. Physiol. 2016, 120, 426–436. [Google Scholar] [CrossRef] [Green Version]
- King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Schmetzer, J.; Schreiber, Y.; Ferreirós, N.; Russe, O.Q.; Geisslinger, G.; Niederberger, E. AMPK contributes to aerobic exercise-induced antinociception downstream of endocannabinoids. Neuropharmacology 2017, 124, 134–142. [Google Scholar] [CrossRef]
- He, J.; Yao, J.; Sheng, H.; Zhu, J. Involvement of the Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A–Alternative Splicing Factor–Calcium/Calmodulin-Dependent Protein Kinase IIδ Signaling Pathway in Myocardial Infarction-Induced Heart Failure of Rats. J. Card. Fail. 2015, 21, 751–760. [Google Scholar] [CrossRef]
- Chu, C.T. Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol. Dis. 2019, 122, 23–34. [Google Scholar] [CrossRef]
- Halestrap, A. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006, 34 Pt 2, 232–237. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Bae, J.H.; Kim, T.N.; Kwak, H.-B.; Kha, P.T.; Han, J. Exercise-Induced Circulating Irisin Level Is Correlated with Improved Cardiac Function in Rats. Int. J. Environ. Res. Public Health 2020, 17, 3863. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef]
- Karnia, M.J.; Myślińska, D.; Dzik, K.; Flis, D.J.; Podlacha, M.; Kaczor, J.J. BST Stimulation Induces Atrophy and Changes in Aerobic Energy Metabolism in Rat Skeletal Muscles—The Biphasic Action of Endogenous Glucocorticoids. Int. J. Mol. Sci. 2020, 21, 2787. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Kou, W.; Xu, X.; Zhou, S.; Luan, P.; Xu, X.; Li, H.; Zhuang, J.; Wang, J.; Zhao, Y. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin. Sci. 2019, 133, 611–627. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, N.; Wang, Y.; Yang, C.; Wang, Y.; Xin, C.; Zhao, J.; Jin, Z.; Cao, F.; Zhang, Z. FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Chen, R.-R.; Fan, X.-H.; Chen, G.; Zeng, G.-W.; Xue, Y.-G.; Liu, X.-T.; Wang, C.-Y. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFβ1/Smad2/3 signaling axis. Chem. Interact. 2019, 302, 11–21. [Google Scholar] [CrossRef]
- Chen, K.; Xu, Z.; Liu, Y.; Wang, Z.; Li, Y.; Xu, X.; Chen, C.; Xia, T.; Liao, Q.; Yao, Y.; et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017, 9, eaao6298. [Google Scholar] [CrossRef] [Green Version]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy Contributes to the Pathogenesis of Inflammatory Diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-M.; Jung, Y.-K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; He, Y.; Luo, X.; Zhang, W.; Yu, L.; Chen, X.; He, X.; Yuan, Y.; Wang, X.; et al. Qiliqiangxin reduced cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1/Parkin-mediated mitochondrial autophagy. BMC Complement. Med. Ther. 2020, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Nazem, S.; Rabiee, F.; Ghaedi, K.; Babashah, S.; Sadeghizadeh, M.; Nasr-Esfahani, M.H. Fndc5 knockdown induced suppression of mitochondrial integrity and significantly decreased cardiac differentiation of mouse embryonic stem cells. J. Cell. Biochem. 2018, 119, 4528–4539. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging 2020, 12, 4474–4488. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, J.-H.; Seo, D.-Y.; Han, J.; Jung, S.-J.; Kwak, H.-B. Effects of Acute Exercise on Mitochondrial Function, Dynamics, and Mitophagy in Rat Cardiac and Skeletal Muscles. Int. Neurourol. J. 2019, 23, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, P.; Chen, Q.; Li, C. Exercise enhances mitochondrial fission and mitophagy to improve myo-pathy following critical limb ischemia in elderly mice via the PGC1a/FNDC5/irisin pathway. Skelet. Muscle 2020, 10, 25. [Google Scholar] [CrossRef]
- Cai, M.; Wang, Q.; Liu, Z.; Jia, D.; Feng, R.; Tian, Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018, 213, 40–49. [Google Scholar] [CrossRef]
- Liang, Q.; Cai, M.; Zhang, J.; Song, W.; Zhu, W.; Xi, L.; Tian, Z. Role of Muscle-Specific Histone Methyltransferase (Smyd1) in Exercise-Induced Cardioprotection against Pathological Remodeling after Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 7010. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Du, J.; Wang, M.H.; Li, J.M.; Yang, B.; Chen, Y.; Dai, J.C.; Zhang, C.; Zhou, J. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/reperfusion. Oxidative Med. Cell. Longev. 2019, 2019, 7857082. [Google Scholar] [CrossRef] [Green Version]
- Batirel, S.; Bozaykut, P.; Altundag, E.M.; Özer, N.K.; Mantzoros, C.S. The effect of Irisin on antioxidant system in liver. Free Radic. Biol. Med. 2014, 75 (Suppl. 1), S16. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Han, Y.; Zhu, H.; Zhou, X.; Tan, T.; Zeng, J.; Zhang, J.; Liu, Y.; Li, Y.; et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J. Cardiovasc. Pharmacol. 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, P.S.; Nassar, S.Z.; Issa, Y.; Zahran, N. Irisin vs. Treadmill Exercise in Post Myocardial Infarction Cardiac Rehabilitation in Rats. Arch. Med. Res. 2019, 50, 44–54. [Google Scholar] [CrossRef]
- Bashar, S.M.; El-Sherbeiny, S.M.S.; Boraie, M.Z. Correlation between the blood level of irisin and the severity of acute myocardial infarction in exercise-trained rats. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Hong, Y.; Yu, S.-H.; Wu, X.-B.; Shyu, W.-C.; Chen, J.-S.; Ting, H.; Yang, A.-L.; Lee, S.-D. Antiapoptotic and mitochondrial biogenetic effects of exercise training on ovariectomized hypertensive rat hearts. J. Appl. Physiol. 2019, 126, 1661–1672. [Google Scholar] [CrossRef]
- Jiang, H.-K.; Wang, Y.-H.; Sun, L.; He, X.; Zhao, M.; Feng, Z.-H.; Yu, X.-J.; Zang, W.-J. Aerobic Interval Training Attenuates Mitochondrial Dysfunction in Rats Post-Myocardial Infarction: Roles of Mitochondrial Network Dynamics. Int. J. Mol. Sci. 2014, 15, 5304–5322. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, Y.; Ogasawara, R.; Tamura, Y.; Fujita, S.; Hatta, H. Effect of electrical stimulation-induced resistance exercise on mitochondrial fission and fusion proteins in rat skeletal muscle. Appl. Physiol. Nutr. Metab. 2015, 40, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Sriwijitkamol, A.; Coletta, D.K.; Wajcberg, E.; Balbontin, G.B.; Reyna, S.M.; Barrientes, J.; Eagan, P.A.; Jenkinson, C.P.; Cersosimo, E.; DeFronzo, R.A.; et al. Effect of Acute Exercise on AMPK Signaling in Skeletal Muscle of Subjects with Type 2 Diabetes: A Time-Course and Dose-Response Study. Diabetes 2007, 56, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huertas, J.R.; Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Nordsborg, N.; Martín-Albo, J.; Rueda, A.; Casuso, R.A. Human muscular mitochondrial fusion in athletes during exercise. FASEB J. 2019, 33, 12087–12098. [Google Scholar] [CrossRef] [Green Version]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, Y.; Liu, X.; Dagda, R.K.; Zhang, Y. How AMPK and PKA Interplay to Regulate Mitochondrial Function and Survival in Models of Ischemia and Diabetes. Oxidative Med. Cell. Longev. 2017, 2017, 4353510. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Qi, J.; Gao, L.; Zhang, J. Role of Exercise on Alleviating Pressure Overload-Induced Left Ventricular Dysfunction and Remodeling via AMPK-Dependent Autophagy Activation. Int. Heart J. 2020, 61, 1022–1033. [Google Scholar] [CrossRef]
- Chen, W.R.; Zhou, Y.J.; Yang, J.Q.; Liu, F.; Wu, X.P.; Sha, Y. Melatonin Attenuates Calcium Deposition from Vascular Smooth Muscle Cells by Activating Mitochondrial Fusion and Mitophagy via an AMPK/OPA1 Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 5298483. [Google Scholar] [CrossRef]
- Andreadou, I.; Benaki, D.; Efentakis, P.; Bibli, S.-I.; Milioni, A.-I.; Papachristodoulou, A.; Zoga, A.; Skaltsounis, A.-L.; Mikros, E.; Iliodromitis, E. The Natural Olive Constituent Oleuropein Induces Nutritional Cardioprotection in Normal and Cholesterol-Fed Rabbits: Comparison with Preconditioning. Planta Med. 2015, 81, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Tsoumani, M.; Georgoulis, A.; Nikolaou, P.-E.; Kostopoulos, I.V.; Dermintzoglou, T.; Papatheodorou, I.; Zoga, A.; Efentakis, P.; Konstantinou, M.; Gikas, E.; et al. Acute administration of the olive constituent, oleuropein, combined with ischemic postconditioning increases myocardial protection by modulating oxidative defense. Free Radic. Biol. Med. 2021, 166, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, R.J.; Teschke, S.R.; Reid, E.B.; Durham, K.K.; Kroetsch, J.T.; Rush, J.W. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J. Hypertens. 2012, 30, 725–733. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, B.; Li, T.; Zhu, Y.; Luo, G.; Jiang, Y.; Tang, F.; Jian, Z.; Xiao, Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int. J. Mol. Med. 2017, 41, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, P.-E.; Boengler, K.; Efentakis, P.; Vouvogiannopoulou, K.; Zoga, A.; Gaboriaud-Kolar, N.; Myrianthopoulos, V.; Alexakos, P.; Kostomitsopoulos, N.; Rerras, I.; et al. Investigating and re-evaluating the role of glycogen synthase kinase 3 beta kinase as a molecular target for cardioprotection by using novel pharmacological inhibitors. Cardiovasc. Res. 2019, 115, 1228–1243. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qin, S.; Liang, Q.; Xi, Y.; Bo, W.; Cai, M.; Tian, Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 2021, 9, 701. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060701
Li H, Qin S, Liang Q, Xi Y, Bo W, Cai M, Tian Z. Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines. 2021; 9(6):701. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060701
Chicago/Turabian StyleLi, Hangzhuo, Shuguang Qin, Qiaoqin Liang, Yue Xi, Wenyan Bo, Mengxin Cai, and Zhenjun Tian. 2021. "Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice" Biomedicines 9, no. 6: 701. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9060701