A Primer on Multimodal Imaging and Cardiology-Radiology Congenital Heart Interface
Abstract
:1. Introduction
2. Types of Noninvasive Multimodality Cardiovascular Imaging
- Echocardiography: Echocardiography is the ideal imaging modality in the pediatric setting and is the most widely used (Table 1). Beyond standard transthoracic echocardiography, fetal echocardiography [18], transesophageal echocardiography [19], intravascular ultrasound, and intracardiac echocardiography are used.
- Cardiac Magnetic Resonance Imaging (CMR): CMR is an adjunct to echocardiography (Table 2) and utilized extensively for imaging congenital heart disease, before (Figure 1) and after surgery, (Figure 2 and Figure 3) [20] and in adults [21]. Evaluating the right ventricle using echocardiography becomes particularly difficult in the adult age [20,22]. CMR has become the reference standard to quantify right ventricular volumes [20,22]. CMR is superior to echocardiography for imaging extracardiac thoracic vasculature, and also for diagnosing myocardial diseases including inflammation, ischemia, scar, and infiltration. It is also an effective tool in the diagnostic work-up of all forms of cardiomyopathies, myocarditis and coronary anomalies (Table 3). However, CMR requires dedicated and trained personnel for image acquisition and interpretration for the best results. It is currently also limited in the assessment of diastolic function. The use of contrast with CMR is limited by patient’s renal function. Children younger than 7 years of age often need general anesthesia for CMR. The limitations of CMR in children with implanted devices are decreasing with newer CMR compatible devices. Finally, stress CMR could potentially be useful in the assessment and risk stratification of congenital or acquired coronary artery disease. CMR compatible catheterization laboratories are emerging, which can reduce the lifelong risk for radiation exposures in patients with congenital heart disease [23].
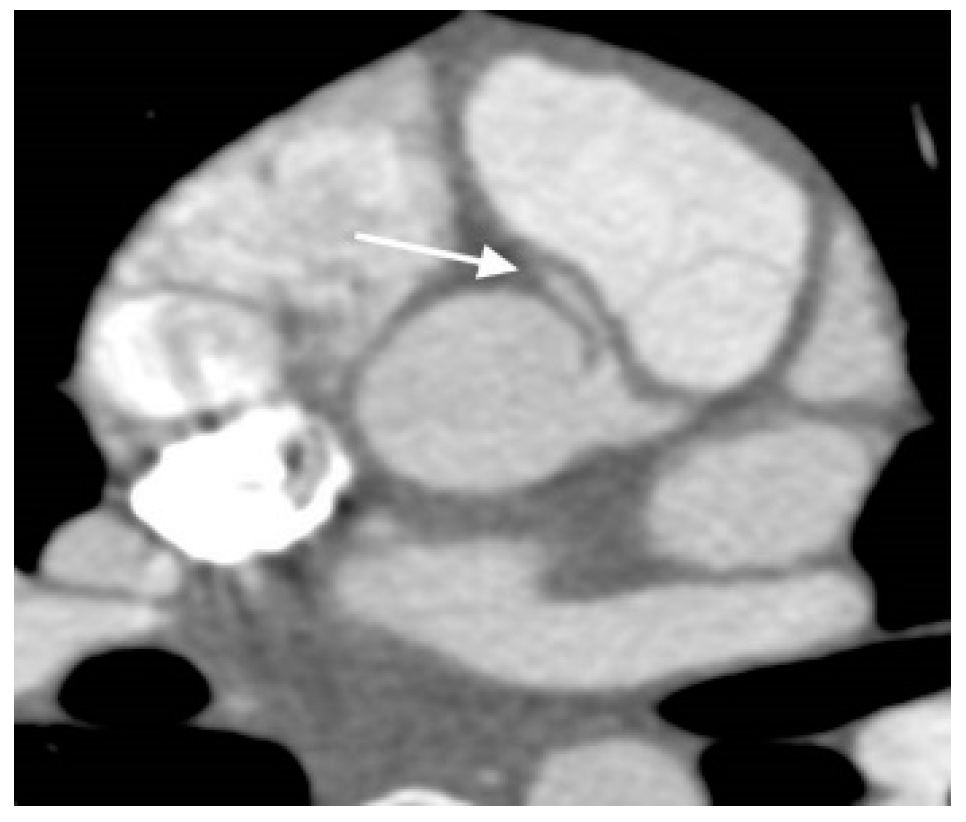
- Cardiac Computed Tomography Angiography (CCTA): The CCTA is valuable for diagnostic assessment of congenital heart disease (Table 4). It causes substantially less radiation exposure compared to cardiac catheterization and can be optimized to very low radiation dosing in moderan scanners [24]. It can also make rapid anatomic assessment of the entire body, in particular the lungs. CCTA has important role in very ill patients, small patients, or in complex anatomy. It is particularly helpful for the assessment of coronary arteries (Figure 4), and extracardiac structures (Figure 5 and Figure 6) including aortic arch, pulmonary veins and pulmonary arteries, as it has one of the best spatial resolutions [25]. CCTA takes much less time and anesthesia than a CMR, and can be performed in the presence of ferromagnetic devices. Other indications include left atrial scar mapping prior to a maze procedure with pulmonary vein isolation, assessment of in-stent luminal stenosis due to intimal hyperplasia, and work-up of athletes for cardiomyopathies (Table 5). The rapid evolution of technology and the ability to make submillimeter volumetric datasets generates exceptionally good quality images at high speed and resolution. Furthermore, computer-assisted calculation of differential lung and blood volumes can be performed.
- Nuclear Imaging: Nuclear imaging of the heart and other organs uses a radiopharmaceutical agent such as thallium-201 and a detection device such as a gamma camera, positron camera, or rectilinear scanner. Clinical applications of cardiac radionuclide imaging are the gated cardiac blood pool scan, myocardial imaging, and detection of myocardial necrosis. Radiation exposure is a significant limitation.
3. Multimodality Imaging Laboratory and Quality Assurance
- Quality Control and Safety: The following aspects are required: (a) Setting standards to ensure the reliability and technical quality of diagnostic images produced; (b) Processes to ensure that equipment and software meet performance specifications; (c) Standards and processes in place to ensure the safety of imaging providers and patients, (d) Certification or accreditation as an independent and transparent evaluation of imaging facilities, and for laboratories to demonstrate accountability and high standards, and (e) Development of mock drills with a check list for all core personnel.
- Appropriate Use Criteria (AUC): AUC defines when and how often it is reasonable to perform a given procedure or test [27]. An appropriate imaging test is one in which the expected incremental information, combined with clinical judgment, exceeds the expected negative consequences by a sufficiently wide margin for a specific indication that the procedure is generally considered acceptable care and a reasonable approach for the indication [27]. Multiple criteria are required to determine AUC, including review of the clinical data, knowledge of the diagnostic tests and procedures, past clinical experiences, and the availability of equipment and/or personnel.
- Layering of Tests: Multiple tests in the same patient can lead to additional costs and lengthy work-flow. Therefore, knowledge of various available modalities and application of the appropriate one for the patient is key [26]. This type of approach need to be developed among the treating physicians.
- Trends in Healthcare and Reimbursement: The rate of imaging volume growth in Medicare has been slowing since 2005 and imaging spending has dropped significantly from previous years [26]. There are ongoing attempts at both the state and national level to eliminate or limit the ability of cardiologists to provide diagnostic imaging services in their offices. The American College of Cardiology strongly supports the ability of specialty physicians, who have knowledge of specific organ systems and disease states, as well as of their patients’ needs, to provide timely and convenient access to imaging services while curtailing costs.
4. Developing a Multimodality Imaging Laboratory
5. Cardiology-Radiology Interface
- Coordinating multimodal imaging services and integrating them to optimize imaging. For example, cardiac 3D and 4D modeling from advanced imaging could be valuable in complex congenital heart disease [29].
- Identifying a cadre of physicians with a congenital heart focus who will regularly attend education programs to share ideas, collaborate and acquire knowledge. The core team members from various modalities with congenital heart focus must be identified and brought together. In that way fresh ideas to improve quality and outcomes would emerge. Team members should be available after hours for emergent imaging on a call schedule, and effectively communicate with the referring providers.
- Developing protocols for image acquisition. The team members should develop, implement and assist in maintaining the required knowledge for protocols and techniques.
- Developing protocols for congenital heart disease reporting using a segmental approach. In this way lesions are not missed on routine studies.
- Delivery of high-quality, post-processed images. Providing clinically relevant visualization and analysis of medical imaging data to patient families and health care providers requesting such services.
- Optimizing image guided interventions in laboratories. Currently transesophageal echocardiogram is routinely used for this purpose in the cardiac catheterization laboratory and in the operating room. CMR using non-ferromagnetic equipment is being used in select laboratories.
6. Avenues for Collaboration and Teaching
- (a)
- Partnering with referring providers: There should be written guidelines in every imaging laboratory which a referring medical provider can use for obtaining an advanced imaging test for their patients.Partnering with ordering clinicians can help reduce inappropriate testing. Electronic Medical Records (EMR) reporting systems should be in compliance with The Intersocietal Commission for the Accreditation of Echocardiography Laboratories (ICAEL), American College of Radiology and so on, as well as understandable to the referring physicians.
- (b)
- Pediatric Cardiology/Adult Cardiology/Radiology/Maternal Fetal Medicine/Emergency Room Ultrasound Trainees: Developing a didactic lecture series for trainees on multimodal imaging. It is important to provide assistance and mentorship to faculty and trainees, including training on new techniques or procedures, research and publications.
- (c)
- Developing a Non-Invasive Cardiac Imaging Fourth-Year Fellowship: Goal of the fellowship is to provide advanced training opportunity in non-invasive cardiac imaging the intent of preparing the advanced trainee for a future faculty position within an academic or a non-academic setting and independently run an imaging lab.
7. Conclusions
Conflicts of Interest
References
- Di Salvo, G.; Miller, O.; Babu Narayan, S.; Li, W.; Budts, W.; Valsangiacomo Buechel, E.R.; Frigiola, A.; van den Bosch, A.E.; Bonello, B.; Mertens, L.; et al. Imaging the adult with congenital heart disease: A multimodality imaging approach-position paper from the EACVI. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1077–1098. [Google Scholar] [CrossRef]
- Prakash, A.; Powell, A.J.; Geva, T. Multimodality noninvasive imaging for assessment of congenital heart disease. Circ. Cardiovasc. Imaging 2010, 3, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Malhotra, M. An approach to imaging adult congenital heart disease: Pitfalls and pearls. Methodist Debakey Cardiovasc. J. 2011, 7, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Valsangiacomo Buechel, E.R.; Grosse-Wortmann, L.; Fratz, S.; Eichhorn, J.; Sarikouch, S.; Greil, G.F.; Beerbaum, P.; Bucciarelli-Ducci, C.; Bonello, B.; Sieverding, L.; et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: An expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Cardiol. Young 2015, 25, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Han, B.K.; Rigsby, C.K.; Hlavacek, A.; Leipsic, J.; Nicol, E.D.; Siegel, M.J.; Bardo, D.; Abbara, S.; Ghoshhajra, B.; Lesser, J.R.; et al. Computed Tomography Imaging in Patients with Congenital Heart Disease Part I: Rationale and Utility. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2015, 9, 475–492. [Google Scholar] [PubMed]
- Han, B.K.; Rigsby, C.K.; Leipsic, J.; Bardo, D.; Abbara, S.; Ghoshhajra, B.; Lesser, J.R.; Raman, S.V.; Crean, A.M.; Nicol, E.D.; et al. Computed Tomography Imaging in Patients with Congenital Heart Disease, Part 2: Technical Recommendations. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2015, 9, 493–513. [Google Scholar]
- Lai, W.W.; Geva, T.; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M.; Pignatelli, R.H.; Rychik, J.; Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. Guidelines and standards for performance of a pediatric echocardiogram: A report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 1413–1430. [Google Scholar]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495, quiz 576–577. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Valverde, I.; Kutty, S. Three-dimensional printed models in congenital heart disease. Int. J. Cardiovasc. Imaging 2017, 33, 137–144. [Google Scholar] [CrossRef]
- Bhat, V.; Belaval, V.; Gadabanahalli, K.; Raj, V.; Shah, S. Illustrated Imaging Essay on Congenital Heart Diseases: Multimodality Approach Part III: Cyanotic Heart Diseases and Complex Congenital Anomalies. J. Clin. Diagn. Res. 2016, 10, TE01–TE10. [Google Scholar] [CrossRef]
- Bhat, V.; Belaval, V.; Gadabanahalli, K.; Raj, V.; Shah, S. Illustrated Imaging Essay on Congenital Heart Diseases: Multimodality Approach Part II: Acyanotic Congenital Heart Disease and Extracardiac Abnormalities. J. Clin. Diagn. Res. 2016, 10, TE01–TE06. [Google Scholar] [CrossRef]
- Bhat, V.; Belaval, V.; Gadabanahalli, K.; Raj, V.; Shah, S. Illustrated Imaging Essay on Congenital Heart Diseases: Multimodality Approach Part I: Clinical Perspective, Anatomy and Imaging Techniques. J. Clin. Diagn. Res. 2016, 10, TE01–TE06. [Google Scholar] [CrossRef]
- Valente, A.M.; Cook, S.; Festa, P.; Ko, H.H.; Krishnamurthy, R.; Taylor, A.M.; Warnes, C.A.; Kreutzer, J.; Geva, T. Multimodality imaging guidelines for patients with repaired tetralogy of fallot: A report from the AmericanSsociety of Echocardiography: Developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J. Am. Soc. Echocardiogr. 2014, 27, 111–141. [Google Scholar] [PubMed]
- Cohen, M.S.; Eidem, B.W.; Cetta, F.; Fogel, M.A.; Frommelt, P.C.; Ganame, J.; Han, B.K.; Kimball, T.R.; Johnson, R.K.; Mertens, L.; et al. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2016, 29, 571–621. [Google Scholar] [PubMed]
- Lee, S.; Uppu, S.C.; Lytrivi, I.D.; Sanz, J.; Weigand, J.; Geiger, M.K.; Shenoy, R.U.; Farooqi, K.; Nguyen, K.H.; Parness, I.A.; et al. Utility of Multimodality Imaging in the Morphologic Characterization of Anomalous Aortic Origin of a Coronary Artery. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 308–317. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Dimopoulos, K.; Budts, W.; Diller, G.P.; Di Salvo, G.; Dellegrottaglie, S.; Festa, P.; Scognamiglio, G.; Rea, G.; Ait Ali, L.; et al. Multimodality imaging in congenital heart disease-related pulmonary arterial hypertension. Heart 2016, 102, 910–918. [Google Scholar] [CrossRef]
- Kindel, S.J.; Hsu, H.H.; Hussain, T.; Johnson, J.N.; McMahon, C.J.; Kutty, S. Multimodality Noninvasive Imaging in the Monitoring of Pediatric Heart Transplantation. J. Am. Soc. Echocardiogr. 2017, 30, 859–870. [Google Scholar]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.J.; Van Der Veld, M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef]
- Puchalski, M.D.; Lui, G.K.; Miller-Hance, W.C.; Brook, M.M.; Young, L.T.; Bhat, A.; Roberson, D.A.; Mercer-Rosa, L.; Miller, O.I.; Parra, D.A.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic: Examination in Children and All Patients with Congenital Heart Disease: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 173–215. [Google Scholar] [CrossRef]
- Fratz, S.; Chung, T.; Greil, G.F.; Samyn, M.M.; Taylor, A.M.; Valsangiacomo Buechel, E.R.; Yoo, S.J.; Powell, A.J. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J. Cardiovasc. Magn. Reson. 2013, 15, 51. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Secinaro, A.; Ciliberti, P.; Fuqua, M.; Nutting, A. Utility of Cardiac Magnetic Resonance Imaging in the Management of Adult Congenital Heart Disease. J. Thorac. Imaging 2017, 32, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Valsangiacomo Buechel, E.R.; Mertens, L.L. Imaging the right heart: The use of integrated multimodality imaging. Eur. Heart J. 2012, 33, 949–960. [Google Scholar] [CrossRef]
- Pushparajah, K.; Tzifa, A.; Bell, A.; Wong, J.K.; Hussain, T.; Valverde, I.; Bellsham-Revell, H.R.; Greil, G.; Simpson, J.M.; Schaeffter, T.; et al. Cardiovascular magnetic resonance catheterization derived pulmonary vascular resistance and medium-term outcomes in congenital heart disease. J. Cardiovasc. Magn. Reson. 2015, 17, 28. [Google Scholar] [CrossRef]
- Young, C.; Taylor, A.M.; Owens, C.M. Paediatric cardiac computed tomography: A review of imaging techniques and radiation dose consideration. Eur. Radiol. 2011, 21, 518–529. [Google Scholar] [CrossRef]
- Kulkarni, A.; Hsu, H.H.; Ou, P.; Kutty, S. Computed Tomography in Congenital Heart Disease: Clinical Applications and Technical Considerations. Echocardiography 2016, 33, 629–640. [Google Scholar] [CrossRef]
- Hendel, R.C. Utilization management of cardiovascular imaging pre-certification and appropriateness. JACC Cardiovasc. Imaging 2008, 1, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M.; Douglas, P.S.; Eidem, B.W.; Lai, W.W.; Lopez, L.; Sachdeva, R. ACC/AAP/AHA/ASE/HRS/SCAI/SCCT/SCMR/SOPE 2014 appropriate use criteria for initial transthoracic echocardiography in outpatient pediatric cardiology: A report of the American College of Cardiology Appropriate Use Criteria Task Force, American Academy of Pediatrics, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J. Am. Coll. Cardiol. 2014, 64, 2039–2060. [Google Scholar]
- Grizzard, J.D. Establishing a cardiac imaging service. J. Am. Coll. Radiol. 2007, 4, 356–361. [Google Scholar] [CrossRef]
- Kutty, S.; Colen, T.M.; Smallhorn, J.F. Three-dimensional echocardiography in the assessment of congenital mitral valve disease. J. Am. Soc. Echocardiogr. 2014, 27, 142–154. [Google Scholar] [CrossRef]
- Hill, K.D.; Frush, D.P.; Han, B.K.; Abbott, B.G.; Armstrong, A.K.; DeKemp, R.A.; Glatz, A.C.; Greenberg, S.B.; Herbert, A.S.; Justino, H.; et al. Radiation Safety in Children With Congenital and Acquired Heart Disease: A Scientific Position Statement on Multimodality Dose Optimization From the Image Gently Alliance. JACC Cardiovasc. Imaging 2017, 10, 797–818. [Google Scholar] [PubMed]





| Advantages of Echocardiography: |
|
|
|
|
|
|
|
| Limitations of Echocardiography: |
|
|
|
|
|
|
| Advantages of CMR over Cardiac Catheterization for Congenital Heart Disease Imaging: |
|
|
|
|
| Advantages of CMR over CTA in Imaging Congenital Heart Disease Are: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Advantages of CCTA over Cardiac Catheterization in Imaging Congenital Heart Disease Are: |
|
|
|
|
|
|
| Advantages of CCTA over CMR in Imaging Congenital Heart Disease Are: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta-Malhotra, M.; Schaaf, W.; Kutty, S. A Primer on Multimodal Imaging and Cardiology-Radiology Congenital Heart Interface. Children 2019, 6, 61. https://0-doi-org.brum.beds.ac.uk/10.3390/children6040061
Gupta-Malhotra M, Schaaf W, Kutty S. A Primer on Multimodal Imaging and Cardiology-Radiology Congenital Heart Interface. Children. 2019; 6(4):61. https://0-doi-org.brum.beds.ac.uk/10.3390/children6040061
Chicago/Turabian StyleGupta-Malhotra, Monesha, William Schaaf, and Shelby Kutty. 2019. "A Primer on Multimodal Imaging and Cardiology-Radiology Congenital Heart Interface" Children 6, no. 4: 61. https://0-doi-org.brum.beds.ac.uk/10.3390/children6040061





