Leveraging Institutional Support to Build an Integrated Multidisciplinary Care Model in Pediatric Inflammatory Bowel Disease
Abstract
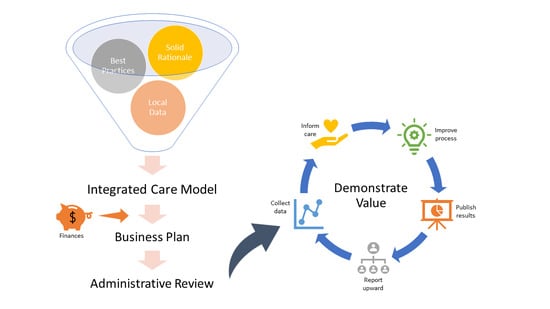
:1. Introduction
2. Making the Case for Inclusion of a Psychologist in IBD Care
3. Justifying an Integrated Model for Delivering Care
4. Addressing Program Finances
5. On-Going Support
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rufo, P.A.; Denson, L.A.; Sylvester, F.A.; Szigethy, E.; Sathya, P.; Lu, Y.; Wahbeh, G.T.; Sena, L.M.; Faubion, W.A. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Mackner, L.M.; Greenley, R.N.; Szigethy, E.; Herzer, M.; Deer, K.; Hommel, K.A. Psychosocial issues in pediatric inflammatory bowel disease: Report of the North America Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Szigethy, E.; Levy-Warren, A.; Whitton, S.; Bousvaros, A.; Gauvreau, K.; Leichtner, A.M.; Beardslee, W.R. Depressive symptoms and inflammatory bowel disease in children and adolescents: A cross-sectional study. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 395–403. [Google Scholar] [CrossRef]
- Murphy, L.K.; de la Vega, R.; Kohut, S.A.; Kawamura, J.S.; Levy, R.L.; Palermo, T.M. Systematic review: Psychosocial correlates of pain in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.K.; Rights, J.D.; Ricciuto, A.; Church, P.C.; Kohut, S.A. Biopsychosocial correlates of presence and intensity of pain in adolescents with inflammatory bowel disease. Front. Pediatr. 2020, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Stapersma, L.; van den Brink, G.; van der Ende, J.; Bodelier, A.G.; van Wering, H.M.; Hurkmans, P.C.W.M.; Mearin, M.L.; van der Meulen-de Jong, A.E.; Escher, J.C.; Utens, E.M.W.J. Illness perceptions and depression are associated with inflammatory bowel disease. Int. J. Behav. Med. 2019, 26, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karwowski, C.A.; Keljo, D.; Szigethy, E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1755–1764. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, R.; Wang, B.; Jiang, K.; Cao, H. Stress triggers flare of inflammatory bowel disease in children and adolescents. Front. Pediatr. 2019, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Szigethy, E.; Murphy, S.M.; Ehrlich, O.G.; Engel-Nitz, N.M.; Heller, C.A.; Henrichsen, K.; Lawton, R.; Meadows, P.; Allen, J.I. Mental health costs of inflammatory bowel diseases. Inflamm. Bowel Dis. 2021, 27, 40–48. [Google Scholar] [CrossRef]
- Johnson, L.E.; Lee, M.J.; Turner-Moore, T.; Grinsted Tate, L.R.; Brooks, A.J.; Tattersall, R.S.; Jones, G.L.; Lobo, A.J. Systematic review of factors affecting transition readiness skill in patients with inflammatory bowel disease. J. Crohns Colitis 2020. [Google Scholar] [CrossRef]
- Plevinsky, J.M.; Wojtowicz, A.A.; Miller, S.A.; Greenley, R.N. Longitudinal barriers to thiopurine adherence in adolescents with inflammatory bowel diseases. J. Pediatr. Psychol. 2019, 44, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Greenley, R.N.; Karazsia, B.; Schurman, J.V.; Gumidyala, A.P.; Nguyen, E.U.; Thomason, M.M.; Walter, J.G.; Noe, J.; Werlin, S.; Kahn, S.A. Trajectories of oral medication adherence in youth with inflammatory bowel disease. Health Psychol. 2015, 34, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mackner, L.M.; Whitaker, B.N.; Maddux, M.H.; Thompson, S.; Hughes-Reid, C.; Drovetta, M.; Reed, B. Depression screening in pediatric inflammatory bowel disease clinics: Recommendations and a toolkit for implementation. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 42–47. [Google Scholar] [CrossRef]
- LeLeiko, N.S.; Lobato, D.; Hagin, S.; McQuaid, E.; Seifer, R.; Kopel, S.J.; Boergers, J.; Nassau, J.; Suorsa, K.; Shapiro, J.; et al. Rates and predictors of oral medication adherence in pediatric IBD patients. Inflamm. Bowel Dis. 2013, 19, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Gray, W.N.; Denson, L.A.; Baldassano, R.N.; Hommel, K.A. Treatment adherence in adolescents with inflammatory bowel disease: The collective impact of barriers to adherence and anxiety/depressive symptoms. J. Pediatr. Psychol. 2012, 37, 282–291. [Google Scholar] [CrossRef]
- Spekhorst, L.M.; Hummel, T.Z.; Benninga, M.A.; van Rheenen, P.F. Adherence to oral maintenance treatment in adolescents with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 264–270. [Google Scholar] [CrossRef]
- Hommel, K.A.; Denson, L.A.; Baldassano, R.N. Oral medication adherence and disease severity in pediatric inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Samson, C.M.; Mager, D.; Frazee, S.; Yu, F. Remission in pediatric inflammatory bowel disease correlates with prescription refill adherence rates. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Szigethy, E.; Kenney, E.; Carpenter, J.; Hardy, D.M.; Fairclough, D.; Bousvaros, A.; Keljo, D.; Weisz, J.; Beardslee, W.R.; Noll, R.; et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1290–1298. [Google Scholar] [CrossRef] [Green Version]
- Keerthy, D.; Youk, A.; Srinath, A.I.; Malas, N.; Bujoreanu, S.; Bousvaros, A.; Keljo, D.; DeMaso, D.R.; Szigethy, E.M. Effect of psychotherapy on healthcare utilization in, 21, 2649–2657.children with inflammatory bowel disease and depression. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Hommel, K.A.; Greenley, R.N.; Maddux, M.H.; Gray, W.N.; Mackner, L.M. Self-management in pediatric inflammatory bowel disease: A clinical report of the North America Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Maddux, M.; Ricks, S.; Delurgio, S.; Hommel, K. A pilot study evaluating the impact of an adherence-promoting intervention among nonadherent youth with inflammatory bowel disease. J. Pediatr. Nurs. 2017, 35, 72–77. [Google Scholar] [CrossRef]
- Hommel, K.A.; Herzer, M.; Ingerski, L.M.; Hente, E.; Denson, L.A. Individually tailored treatment of medication nonadherence. J. Pediatr. Gastroenterol. Hepatol. 2011, 53, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenley, R.N.; Gumidyala, A.P.; Nguyen, E.; Plevinsky, J.M.; Poulopoulos, N.; Thomason, M.M.; Walter, J.G.; Wojtowicz, A.A.; Blank, E.; Gokhale, R.; et al. Can you teach a teen new tricks? Problem solving skills training improves oral medication adherence in pediatric patients with inflammatory bowel disease participating in a randomized trial. Inflamm. Bowel Dis. 2015, 21, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.R.; Kamphaus, R.W. Behavior Assessment System for Children, 3rd ed.; NCS Pearson, Inc.: Bloomington, IN, USA, 2015. [Google Scholar]
- Varni, J.W.; Seid, M.; Kurtin, P.S. The PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Maddux, M.H.; Bass, J.A.; Geraghty-Sirridge, C.; Carpenter, E.; Christenson, K. Assessing psychosocial functioning among youth with newly diagnosed Inflammatory Bowel Disease (IBD): An integrated multidisciplinary clinic approach. Clin. Pract. Pediatr. Psychol. 2013, 1, 333–343. [Google Scholar] [CrossRef]
- Pai, A.L.; Gray, E.; Kurivial, K.; Ross, J.; Schoborg, D.; Goebel, J. The Allocation of Treatment Responsibility scale: A novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr. Transpl. 2010, 14, 993–999. [Google Scholar] [CrossRef]
- Richardson, L.P.; McCauley, E.; Grossman, D.C.; Mccarty, C.A.; Richards, J.; Russo, J.E.; Rockhill, C.; Katon, W. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics 2010, 126, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Keefer, L.; Kiebles, J.L.; Taft, T.H. The role of self-efficacy in inflammatory bowel disease management: Preliminary validation of a disease-specific measure. Inflamm. Bowel Dis. 2011, 17, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Maddux, M.H.; Drovetta, M.; Hasenkamp, R.; Goyal, A.; McCullough, J.; Bass, J. Using a mixed-method approach to develop a transition program for young adults with Inflammatory Bowel disease (IBD). J. Pediatr. Gastroenterol. Nutr. 2019, 70, 195–199. [Google Scholar] [CrossRef]
- Morar, P.; Read, J.; Arora, S.; Hart, A.; Warusavitarne, J.; Green, J.; Sevdalis, N.; Edwards, C.; Faiz, O. Defining the optimal design of the inflammatory bowel disease multidisciplinary team: Results from a multicentre qualitative expert-based study. Frontline Gastroenterol. 2015, 6, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Fiorino, G.; Allocca, M.; Chaparro, M.; Coenen, S.; Fidalgo, C.; Younge, L.; Gisbert, J.P. Quality of care standards in inflammatory bowel disease: A systematic review. J. Crohns Colitis 2019, 13, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kapasi, R.; Glatter, J.; Lamb, C.A.; Acheson, A.G.; Andrews, C.; Arnott, I.D.; Barrett, K.J.; Bell, G.; Bhatnagar, G.; Bloom, S.; et al. Consensus standards of healthcare for adults and children with inflammatory bowel disease in the UK. Frontline Gastroenterol. 2019, 11, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfeld, R.; Nguyen, G.C.; Bernstein, C.N. Integrated care models: Optimizing adult ambulatory care in inflammatory bowel disease. J. Can. Assoc. Gastroenterol. 2020, 3, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Cushing, C.C.; Friesen, C.A.; Schurman, J.V. Collaboration with medical professionals in clinical practice: Pediatric abdominal pain as a test example. Fam. Syst. Health 2012, 30, 279–290. [Google Scholar] [CrossRef]
- Young, N.D.; Mathews, B.L.; Pan, A.Y.; Herndon, J.L.; Bleck, A.A.; Takala, C.R. Warm handoff, or cold shoulder? An analysis of handoffs for primary care behavioral health consultation on patient engagement and systems utilization. Clin. Pract. Pediatr. Psychol. 2020, 8, 241–246. [Google Scholar] [CrossRef]
- Lamparyk, K.; Debeljak, A.; Aylward, L.; Mahajan, L. Impact of integrated care in a pediatric gastroenterology clinic on psychology utilization. Clin. Pract. Pediatr. Psychol. 2018, 6, 51–60. [Google Scholar] [CrossRef]
- Patel, K.; Presser, E.; George, M.; McClellan, M. Shifting away from fee-for-service: Alternative approaches to payment in gastroenterology. Clin. Gastroenterol. Hepatol. 2016, 14, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Goldstein-Leever, A.; Bass, J.A.; Goyal, A.; Maddux, M.H. Health-related quality of life predicts psychology referral in youth with inflammatory bowel disease. J. Pediatr. Nurs. 2019, 47, 73–77. [Google Scholar] [CrossRef]
- Maddux, M.H.; Ricks, S.; Bass, J. Patient and caregiver perspectives on transition and transfer. Clin. Pediatr. 2017, 56, 278–283. [Google Scholar] [CrossRef]
- Schurman, J.V.; Cushing, C.C.; Carpenter, E.; Christenson, K. Volitional and accidental nonadherence to pediatric inflammatory bowel disease treatment plans: Initial investigation of associations with quality of life and disease activity. J. Pediatr. Psychol. 2011, 36, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Maddux, M.; Gordy, A.; Schurman, C.; Cole, T.; Staggs, V. Initial validation of IBD KNOW-IT: Measuring patient and caregiver knowledge of a child’s disease and treatment regimen. J. Clin. Psychol. Med. Settings 2020, 27, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Schurman, J.V.; Friesen, C.A.; Dai, H.; Danda, C.E.; Hyman, P.E.; Cocjin, J.T. Sleep problems and functional disability in children with functional gastrointestinal disorders: An examination of the potential mediating effects of physical and emotional symptoms. BMC Gastroenterol. 2012, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Cushing, C.C.; Kichline, T.; Friesen, C.; Schurman, J.V. Individual differences in the relationship between pain fear, avoidance, and pain severity in a chronic abdominal pain sample and the moderating effect of child age. Ann. Behav. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schurman, J.V.; Singh, M.; Singh, V.; Neilan, N.; Friesen, C.A. Symptoms and subtypes in pediatric functional dyspepsia: Relation to mucosal inflammation and psychological functioning. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, V.; Schurman, J.V.; Colombo, J.M.; Friesen, C.A. The relationship between mucosal inflammatory cells, specific symptoms, and psychological functioning in youth with irritable bowel syndrome. Sci. Rep. 2020, 10, 11988. [Google Scholar] [CrossRef] [PubMed]
- Deacy, A.D.; Friesen, C.A.; Staggs, V.S.; Schurman, J.V. Evaluation of clinical outcomes in an interdisciplinary abdominal pain clinic: A retrospective, exploratory review. World J. Gastroenterol. 2019, 25, 3079–3090. [Google Scholar] [CrossRef]
- Ryan, J.L.; Dandridge, L.M.; Fischer, R.T. Adherence to laboratory testing in pediatric liver transplant recipients. Pediatr. Transpl. 2020. [Google Scholar] [CrossRef]
- Schurman, J.V.; Friesen, C.A. Integrative treatment approaches: Family satisfaction with a multidisciplinary pediatric abdominal pain clinic. Int. J. Integr. Care 2010, 10, e51. [Google Scholar] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurman, J.V.; Friesen, C.A. Leveraging Institutional Support to Build an Integrated Multidisciplinary Care Model in Pediatric Inflammatory Bowel Disease. Children 2021, 8, 286. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040286
Schurman JV, Friesen CA. Leveraging Institutional Support to Build an Integrated Multidisciplinary Care Model in Pediatric Inflammatory Bowel Disease. Children. 2021; 8(4):286. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040286
Chicago/Turabian StyleSchurman, Jennifer Verrill, and Craig A. Friesen. 2021. "Leveraging Institutional Support to Build an Integrated Multidisciplinary Care Model in Pediatric Inflammatory Bowel Disease" Children 8, no. 4: 286. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040286