Risperidone or Aripiprazole Can Resolve Autism Core Signs and Symptoms in Young Children: Case Study
Abstract
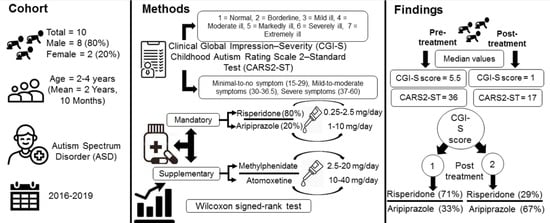
:1. Introduction
2. Patients and Methods
2.1. Patients and Data Collection
2.2. Diagnosis
2.3. Treatment
2.4. Monitoring for Safety
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. DSM-5 Task Force. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Centers for Disease Control. Data & Statistics on Autism Spectrum Disorder. 2018. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 18 December 2020).
- James, S.N.; Smith, C.J. Early Autism Diagnosis in the Primary Care Setting. Semin. Pediatr. Neurol. 2020, 35, 100827. [Google Scholar] [CrossRef]
- Lai, M.-C.; Anagnostou, E.; Wiznitzer, M.; Allison, C.; Baron-Cohen, S. Evidence-based support for autistic people across the lifespan: Maximising potential, minimising barriers, and optimising the person–environment fit. Lancet Neurol. 2020, 19, 434–451. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bauman, M.L.; Choueiri, R.; Kasari, C.; Carter, A.S.; Granpeesheh, D.; Mailloux, Z.; Roley, S.S.; Wagner, S.; Fein, D.; et al. Early Intervention for Children with Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research. Pediatrics 2015, 136 (Suppl. 1), S60–S81. [Google Scholar] [CrossRef] [Green Version]
- Carbone, P.S.; Campbell, K.; Wilkes, J.; Stoddard, G.J.; Huynh, K.; Young, P.C.; Gabrielsen, T.P. Primary Care Autism Screening and Later Autism Diagnosis. Pediatrics 2020, 146, e20192314. [Google Scholar] [CrossRef]
- Magiati, I.; Tay, X.W.; Howlin, P. Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: A systematic review of longitudinal follow-up studies in adulthood. Clin. Psychol. Rev. 2014, 34, 73–86. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Seltzer, M.M.; Lam, K.S.L.; Bodfish, J.W. Age-Related Differences in Restricted Repetitive Behaviors in Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Bal, V.H.; Kim, S.-H.; Fok, M.; Lord, C. Autism spectrum disorder symptoms from ages 2 to 19 years: Implications for diagnosing adolescents and young adults. Autism Res. 2019, 12, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Militerni, R.; Bravaccio, C.; Falco, C.; Fico, C.; Palermo, M.T. Repetitive behaviors in autistic disorder. Eur. Child Adolesc. Psychiatry 2002, 11, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E. Clinical trials in autism spectrum disorder: Evidence, challenges and future directions. Curr. Opin. Neurol. 2018, 31, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, F.; Siegel, M.; Woodbury-Smith, M.; King, B.; McCracken, J.; State, M. Practice Parameter for the Assessment and Treatment of Children and Adolescents with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 237–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, S.L.; Levy, S.E.; Myers, S.M.; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, Evaluation, and Management of Children with Autism Spectrum Disorder. Pediatrics 2019, 145, e20193447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Food and Drug Administration. Approval for Risperdal (Risperidone) in Treatment of the Irritability Associated with Autistic Disorder. 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/020272Orig1s036,s041,020588Orig1s024,s028,s029,21444Orig1s008,s015.pdf (accessed on 18 December 2020).
- Bristol Myers Squibb. U.S. Food and Drug Administration Approves ABILIFY® (Aripiprazole) for the Treatment of Irritability Associated with Autistic Disorder in Pediatric Patients (Ages 6 to 17 Years). 2009. Available online: https://news.bms.com/news/details/2009/US-Food-and-Drug-Administration-Approves-ABILIFY-aripiprazole-for-the-Treatment-of-Irritability-Associated-with-Autistic-Disorder-in-Pediatric-Patients-Ages-6-to-17-Years/default.aspx (accessed on 18 December 2020).
- Kent, J.M.; Kushner, S.; Ning, X.; Karcher, K.; Ness, S.; Aman, M.; Singh, J.; Hough, D. Risperidone Dosing in Children and Adolescents with Autistic Disorder: A Double-Blind, Placebo-Controlled Study. J. Autism Dev. Disord. 2013, 43, 1773–1783. [Google Scholar] [CrossRef]
- Owen, R.; Sikich, L.; Marcus, R.N.; Corey-Lisle, P.; Manos, G.; McQuade, R.D.; Carson, W.H.; Findling, R.L. Aripiprazole in the Treatment of Irritability in Children and Adolescents with Autistic Disorder. Pediatrics 2009, 124, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, M.; Pedapati, E.V.; Dominick, K.L.; Wink, L.K.; Erickson, C.A. Recent Advances in the Pharmacological Management of Behavioral Disturbances Associated with Autism Spectrum Disorder in Children and Adolescents. Pediatr. Drugs 2020, 22, 473–483. [Google Scholar] [CrossRef]
- Fung, L.K.; Mahajan, R.; Nozzolillo, A.; Bernal, P.; Krasner, A.; Jo, B.; Coury, D.; Whitaker, A.; Veenstra-VanderWeele, J.; Hardan, A.Y. Pharmacologic Treatment of Severe Irritability and Problem Behaviors in Autism: A Systematic Review and Meta-analysis. Pediatrics 2016, 137 (Suppl. 2), S124–S135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccia, S. Safety and Pharmacokinetics of Atypical Antipsychotics in Children and Adolescents. Pediatr. Drugs 2013, 15, 217–233. [Google Scholar] [CrossRef]
- Politte, L.C.; McDougle, C.J. Atypical antipsychotics in the treatment of children and adolescents with pervasive developmental disorders. Psychopharmacology 2014, 231, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Aman, M.G.; Handen, B.L.; Sanders, K.B.; Shui, A.; Hollway, J.A.; Brian, J.; Arnold, L.E.; Capano, L.; Hellings, J.A.; et al. Metformin for Treatment of Overweight Induced by Atypical Antipsychotic Medication in Young People with Autism Spectrum Disorder: A randomized clinical trial. JAMA Psychiatry 2016, 73, 928–937. [Google Scholar] [CrossRef]
- Sturman, N.; Deckx, L.; Van Driel, M.L. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst. Rev. 2017, 11, CD011144. [Google Scholar] [CrossRef]
- Handen, B.L.; Aman, M.G.; Arnold, L.E.; Hyman, S.L.; Tumuluru, R.V.; Lecavalier, L.; Corbett-Dick, P.; Pan, X.; Hollway, J.A.; Buchan-Page, K.A.; et al. Atomoxetine, Parent Training, and Their Combination in Children with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scahill, L.; McCracken, J.T.; King, B.H.; Rockhill, C.; Shah, B.; Politte, L.; Sanders, R.; Minjarez, M.; Cowen, J.; Mullett, J.; et al. Extended-Release Guanfacine for Hyperactivity in Children with Autism Spectrum Disorder. Am. J. Psychiatry 2015, 172, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, Q.X. A Systematic Review of the Use of Bupropion for Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. J. Child Adolesc. Psychopharmacol. 2017, 27, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Schwam, J.S.; Klass, E.; Alonso, C.; Perry, R. RISPERIDONE AND REFUSAL TO EAT. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 572–573. [Google Scholar] [CrossRef]
- Posey, D.J.; Walsh, K.H.; Wilson, G.A.; McDougle, C.J. Risperidone in the Treatment of Two Very Young Children with Autism. J. Child Adolesc. Psychopharmacol. 1999, 9, 273–276. [Google Scholar] [CrossRef]
- Masi, G.; Cosenza, A.; Mucci, M.; De Vito, G. Risperidone Monotherapy in Preschool Children with Pervasive Developmental Disorders. J. Child Neurol. 2001, 16, 395–400. [Google Scholar] [CrossRef]
- Masi, G.; Cosenza, A.; Mucci, M.; Brovedani, P. Open Trial of Risperidone in 24 Young Children with Pervasive Developmental Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1206–1214. [Google Scholar] [CrossRef]
- Boon-Yasidhi, V.; Tarugsa, J.; Suwanwattana, C.; Soising, L. Risperidone in the treatment of autistic Thai children under 4 years of age. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2002, 85 (Suppl. 2), S784–S789. [Google Scholar]
- Diler, R.S.; Firat, S.; Avci, A.; Fırat, S.; Avcı, A. An open-label trial of risperidone in children with autism. Curr. Ther. Res. 2002, 63, 91–102. [Google Scholar] [CrossRef]
- Masi, G.; Cosenza, A.; Mucci, M.; Brovedani, P. A 3-Year Naturalistic Study of 53 Preschool Children with Pervasive Developmental Disorders Treated With Risperidone. J. Clin. Psychiatry 2003, 64, 1039–1047. [Google Scholar] [CrossRef]
- Nagaraj, R.; Singhi, P.; Malhi, P. Risperidone in Children With Autism: Randomized, Placebo-Controlled, Double-Blind Study. J. Child Neurol. 2006, 21, 450–455. [Google Scholar] [CrossRef]
- Boon-Yasidhi, V.; Jearnarongrit, P.; Tulayapichitchock, P.; Tarugsa, J. Adverse Effects of Risperidone in Children with Autism Spectrum Disorders in a Naturalistic Clinical Setting at Siriraj Hospital, Thailand. Psychiatry J. 2014, 2014, 136158. [Google Scholar] [CrossRef]
- Fayyazi, A.; Salari, E.; Khajeh, A.; Gajarpour, A. A Comparison of Risperidone and Buspirone for Treatment of Behavior Disorders in Children with Phenylketonuria. Iran. J. Child Neurol. 2014, 8, 33–38. [Google Scholar]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Dawkins, T.; Meyer, A.T.; Van Bourgondien, M.E. The Relationship between the Childhood Autism Rating Scale: Second Edition and Clinical Diagnosis Utilizing the DSM-IV-TR and the DSM-5. J. Autism Dev. Disord. 2016, 46, 3361–3368. [Google Scholar] [CrossRef]
- Schopler, E.; Van Bourgondien, M.E.; Wellman, G.J.; Love, S.R. Childhood Autism Rating Scale, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2010. [Google Scholar]
- Yilmaz, S.; Serdaroglu, G.; Akcay, A.; Gökben, S. Clinical characteristics and outcome of children with electrical status epilepticus during slow wave sleep. J. Pediatr. Neurosci. 2014, 9, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hergüner, M.Ö.; Incecik, F.; Altunbaşak, S.; Kiriş, N. Clinical Characteristics of 10 Patients with Continuous Spikes and Waves During Slow Sleep Syndrome. Pediatr. Neurol. 2008, 38, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Brian, J.A.; Zwaigenbaum, L.; Ip, A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr. Child Health 2019, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Nachshen, J.; Garcin, N.; Moxness, K.; Tremblay, Y.; Hutchinson, P.; Lachance, A.; Beaurivage, M.; Breitenbach, M.; Bryson, S.; Burack, J.; et al. Screening, Assessment, and Diagnosis of Autism Spectrum Disorders in Young Children: Canadian Best Practice Guidelines; Miriam Foundation: Montreal, QC, Canada, 2008. [Google Scholar]
- Alsayouf, H.A.; Talo, H.; Biddappa, M.L.; Qasaymeh, M.; Qasem, S.; Reyes, E.D.L. Pharmacological Intervention in Children with Autism Spectrum Disorder with Standard Supportive Therapies Significantly Improves Core Signs and Symptoms: A Single-Center, Retrospective Case Series. Neuropsychiatr. Dis. Treat. 2020, 16, 2779–2794. [Google Scholar] [CrossRef]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care 2004, 27, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.; Panagiotopoulos, C.; McCrindle, B.; Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA) Guideline Group. Management recommendations for metabolic complications associated with second generation antipsychotic use in children and youth. Paediatr. Child Health 2011, 16, 575–580. [Google Scholar]
- Kimura, G.; Kadoyama, K.; Brown, J.B.; Nakamura, T.; Miki, I.; Nisiguchi, K.; Sakaeda, T.; Okuno, Y. Antipsychotics-Associated Serious Adverse Events in Children: An Analysis of the FAERS Database. Int. J. Med. Sci. 2015, 12, 135–140. [Google Scholar] [CrossRef]
- Roke, Y.; Van Harten, P.N.; Boot, A.M.; Buitelaar, J.K. Antipsychotic Medication in Children and Adolescents: A Descriptive Review of the Effects on Prolactin Level and Associated Side Effects. J. Child Adolesc. Psychopharmacol. 2009, 19, 403–414. [Google Scholar] [CrossRef]
- Haddad, P.M.; Wieck, A. Antipsychotic-Induced Hyperprolactinaemia: Mechanisms, clinical features and management. Drugs 2004, 64, 2291–2314. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Manu, P.; Olshanskiy, V.; Napolitano, B.; Kane, J.M.; Malhotra, A.K. Cardiometabolic Risk of Second-Generation Antipsychotic Medications during First-Time Use in Children and Adolescents. JAMA 2009, 302, 1765–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.G.; Juul, K.; Fink-Jensen, A.; Correll, C.U.; Pagsberg, A.K. Corrected QT Changes during Antipsychotic Treatment of Children and Adolescents: A Systematic Review and Meta-Analysis of Clinical Trials. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 25–36. [Google Scholar] [CrossRef]
- Rizzo, R.; Gulisano, M.; Calì, P.V.; Di Pino, A. Mandatory electrocardiographic monitoring in young patients treated with psychoactive drugs. Eur. Child Adolesc. Psychiatry 2013, 22, 577–579. [Google Scholar] [CrossRef]
- Asakawa, T.; Sugiyama, K.; Nozaki, T.; Sameshima, T.; Kobayashi, S.; Wang, L.; Hong, Z.; Chen, S.-J.; Li, C.-D.; Ding, D.; et al. Current behavioral assessments of movement disorders in children. CNS Neurosci. Ther. 2018, 24, 863–875. [Google Scholar] [CrossRef]
- Hashemi, E.; Ariza, J.; Rogers, H.; Noctor, S.C.; Martinez-Cerdeno, V. The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cereb. Cortex 2017, 27, 1931–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, J.; Ejaz, E.; Ariza, J.; Noctor, S.C.; Martínez-Cerdeño, V. RELN-expressing neuron density in layer I of the superior temporal lobe is similar in human brains with autism and in age-matched controls. Neurosci. Lett. 2014, 579, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.; Treiber, J.M.; Shukla, D.K.; Shih, P.; Müller, R.-A. Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain 2013, 136, 1942–1955. [Google Scholar] [CrossRef]
- Cheon, K.-A.; Kim, Y.-S.; Oh, S.-H.; Park, S.-Y.; Yoon, H.-W.; Herrington, J.; Nair, A.; Koh, Y.-J.; Jang, D.-P.; Leventhal, B.L.; et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Res. 2011, 1417, 77–86. [Google Scholar] [CrossRef]
- Chirdkiatgumchai, V.; Xiao, H.; Fredstrom, B.K.; Adams, R.E.; Epstein, J.N.; Shah, S.S.; Brinkman, W.B.; Kahn, R.S.; Froehlich, T.E. National Trends in Psychotropic Medication Use in Young Children: 1994–2009. Pediatrics 2013, 132, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pringsheim, T.; Stewart, D.G.; Chan, P.; Tehrani, A.; Patten, S.B. The Pharmacoepidemiology of Psychotropic Medication Use in Canadian Children from 2012 to 2016. J. Child Adolesc. Psychopharmacol. 2019, 29, 740–745. [Google Scholar] [CrossRef]
- Fanton, J.; Gleason, M.M. Psychopharmacology and Preschoolers: A Critical Review of Current Conditions. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.M.; Egger, H.L.; Emslie, G.J.; Greenhill, L.L.; Kowatch, R.A.; Lieberman, A.F.; Luby, J.L.; Owens, J.; Scahill, L.D.; Scheeringa, M.S.; et al. Psychopharmacological Treatment for Very Young Children: Contexts and Guidelines. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1532–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brophy, S.; Kennedy, J.; Fernandez-Gutierrez, F.; John, A.; Potter, R.; Linehan, C.; Kerr, M. Characteristics of Children Prescribed Antipsychotics: Analysis of Routinely Collected Data. J. Child Adolesc. Psychopharmacol. 2018, 28, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Lòpez-De Fede, A.; Vyavaharkar, M.; Bellinger, J.D. Antipsychotic prescriptions for children aged 5 years or younger: Do we need policy oversight standards? SAGE Open 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Luby, J.; Mrakotsky, C.; Stalets, M.M.; Belden, A.; Heffelfinger, A.; Williams, M.; Spitznagel, E. Risperidone in Preschool Children with Autistic Spectrum Disorders: An Investigation of Safety and Efficacy. J. Child Adolesc. Psychopharmacol. 2006, 16, 575–587. [Google Scholar] [CrossRef]
- Persico, A.M.; Arango, C.; Buitelaar, J.K.; Correll, C.U.; Glennon, J.C.; Hoekstra, P.J.; Moreno, C.; Vitiello, B.; Vorstman, J.; Zuddas, A. Unmet needs in paediatric psychopharmacology: Present scenario and future perspectives. Eur. Neuropsychopharmacol. 2015, 25, 1513–1531. [Google Scholar] [CrossRef]
- Olfson, M.; Crystal, S.; Huang, C.; Gerhard, T. Trends in antipsychotic drug use by very young, privately insured children. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.N.; Cluxton-Keller, F.; Gross, D. Antipsychotic Medication Prescribing Trends in Children and Adolescents. J. Pediatr. Health Care 2012, 26, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Cicala, G.; Barbieri, M.A.; Santoro, V.; Tata, C.; Colucci, P.V.; Vanadia, F.; Drago, F.; Russo, C.; Cutroneo, P.M.; Gagliano, A.; et al. Safety and Tolerability of Antipsychotic Drugs in Pediatric Patients: Data From a 1-Year Naturalistic Study. Front. Psychiatry 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougle, C.J.; Scahill, L.; Aman, M.G.; McCracken, J.T.; Tierney, E.; Davies, M.; Arnold, L.E.; Posey, D.J.; Martin, A.; Ghuman, J.K.; et al. Risperidone for the Core Symptom Domains of Autism: Results from the Study by the Autism Network of the Research Units on Pediatric Psychopharmacology. Am. J. Psychiatry 2005, 162, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Sex | Age (Years); Date Medication Started (mm/yy) | Medication Starting Dose (mg) | Maximum Dose (mg); a Time to Reach this Dose | Current Dose (mg); a Total Duration | Laboratory Findings b | Cardiac Evaluation | Medication and Side Effects |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 2; 06/19 | Risperidone 0.25 mg at night. | Risperidone 2 mg in the morning and 0.5 mg at night; 8 months. | Risperidone 2 mg in the morning and 0.5 mg at night; 15 months. | Normal | Normal | Currently weaning off risperidone. |
| 2 | F | 3; 03/19 | Aripiprazole 1 mg at night. Atomoxetine 10 mg added at night after 1 month. Methylphenidate 2.5 mg added in the morning after 3 months. | Aripiprazole 4 mg at night; 1 month. Atomoxetine 40 mg at night; 3 months. Methylphenidate 2.5 mg in the morning; 3 days. | Aripiprazole 4 mg at night; 21 months. Atomoxetine 40 mg at night; 18 months. Methylphenidate 2.5 mg in the morning; 16 months. | Normal | Normal | Weight gain on aripiprazole; therefore, atomoxetine, and later methylphenidate, were added. Methylphenidate was tried for 3 days at 5 mg but caused irritability so it was reduced back to 2.5 mg. Currently weaning off aripiprazole. |
| 3 | M | 3; 06/19 | Risperidone 0.25 mg at night. Methylphenidate 2.5 mg added in the morning after 3 months. | Risperidone 2 mg in the morning and 0.5 mg at night; 9 months. Methylphenidate 10 mg in the morning and at 2 pm; 5 months. | Risperidone 2 mg in the morning and 0.5 mg at night; 15 months. Methylphenidate 10 mg in the morning and at 2 pm; 12 months. | Normal | Normal | Weight gain on risperidone; methylphenidate was added later to control weight and help attention. |
| 4 | M | 3; 09/19 | Aripiprazole 1 mg at night. | Aripiprazole 10 mg at night; 7 months. Atomoxetine 10 mg at night; one week. Methylphenidate 2.5 mg in the morning; 3 days. | Aripiprazole 10 mg at night; 15 months. | Normal | Normal | One-week atomoxetine 10 mg trial made him very aggressive. Three-day methylphenidate 2.5 mg trial made him irritable. |
| 5 | M | 3; 07/19 | Risperidone 0.25 mg at night. Aripiprazole 1 mg added at night after 5 months. | Risperidone 1.5 mg twice a day; 5 months. Aripiprazole 3 mg at night; 2 months. | Aripiprazole 3 mg at night; 12 months. | Normal | Normal | Risperidone was weaned after 5 months as there was no clear improvement. It was replaced by aripiprazole. |
| 6 | M | 2⅓; 09/17 | Risperidone 0.25 mg at night. | Risperidone 1.75 mg in the morning and 0.5 mg at night; 13 months. He was subsequently weaned off risperidone and was on risperidone for a total of 28 months. | None | Normal | Normal | |
| 7 | M | 2; 12/19 | Risperidone 0.25 mg at night. | Risperidone 1 mg in the morning and 0.75 mg at night; 4 months. | Risperidone 1 mg in the morning and 0.75 mg at night; 12 months. | Normal | Normal | |
| 8 | M | 3½; 09/17 | Risperidone 0.25 mg at night. | Risperidone 2 mg in the morning and 0.5 mg at night; 8 months. He was subsequently weaned off risperidone and was on risperidone for a total of 20 months. | None | Prolactin was elevated 6-fold with no clinical signs or symptoms of hyperprolactinemia. | Normal | Mild weight gain. |
| 9 | F | 3⅓; 12/16 | Risperidone 0.25 mg at night. Atomoxetine 10 mg added at night after 9 months. | Risperidone 1.5 mg in the morning and 0.75 mg at night; 12 months. She was subsequently weaned off risperidone and was on risperidone for a total of 27 months. Atomoxetine 40 mg; 2 months. | Atomoxetine 40 mg at night only; 48 months. | Prolactin was elevated 2.5-fold with no clinical signs or symptoms of hyperprolactinemia. | Normal | Once she attained normal development and complete resolution of her ASD symptoms she was weaned off risperidone. Kept on atomoxetine to help control hyperactivity only. |
| 10 | M | 3; 07/18 | Risperidone 0.25 mg at night. Atomoxetine 10 mg added at night after 10 months. Methylphenidate 2.5 mg added in the morning after 11 months. | Risperidone 2 mg in the morning and 0.5 mg at night; 24 months. Atomoxetine 10 mg at night stopped after one week due to irritability. Methylphenidate 2.5 mg in the morning stopped after one week due to irritability. | None | Normal | Normal |
| Case No. | Diagnosis: DSM-5 Criteria | CGI-S/CARS2-ST Scores before Treatment | CGI-S/CARS2-ST Scores after Treatment | CGI-I Score after Treatment | Current Status |
|---|---|---|---|---|---|
| 1 | ASD level 2 | 5/36 | 1/17 | 1 | Complete resolution of ASD symptoms, currently weaning off medications |
| 2 | ASD level 3 | 6/45.5 | 1/16 | 1 | Complete resolution of ASD symptoms, currently weaning off medications |
| 3 | ASD level 2 | 6/45 | 2/23 | 2 | Much improved |
| 4 | ASD level 2 | 5/39.5 | 2/21 | 2 | Much improved |
| 5 | ASD level 2 | 5/36 | 2/18.5 | 2 | Much improved |
| 6 | ASD level 3 | 6/42 | 1/16 | 1 | Complete resolution of ASD symptoms, off all medications |
| 7 | ASD level 3 | 6/41 | 2/21 | 2 | Much improved |
| 8 | ASD level 2 | 5/36 | 1/15 | 1 | Complete resolution of ASD symptoms, off all medications |
| 9 | ASD level 2 | 5/36 | 1/17 | 1 | Complete resolution of ASD symptoms, only on atomoxetine to control hyperactivity |
| 10 | ASD level 2 | 6/36.5 | 1/15 | 1 | Complete resolution of ASD symptoms, off all medications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayouf, H.A.; Talo, H.; Biddappa, M.L.; De Los Reyes, E. Risperidone or Aripiprazole Can Resolve Autism Core Signs and Symptoms in Young Children: Case Study. Children 2021, 8, 318. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050318
Alsayouf HA, Talo H, Biddappa ML, De Los Reyes E. Risperidone or Aripiprazole Can Resolve Autism Core Signs and Symptoms in Young Children: Case Study. Children. 2021; 8(5):318. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050318
Chicago/Turabian StyleAlsayouf, Hamza A., Haitham Talo, Marisa L. Biddappa, and Emily De Los Reyes. 2021. "Risperidone or Aripiprazole Can Resolve Autism Core Signs and Symptoms in Young Children: Case Study" Children 8, no. 5: 318. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050318