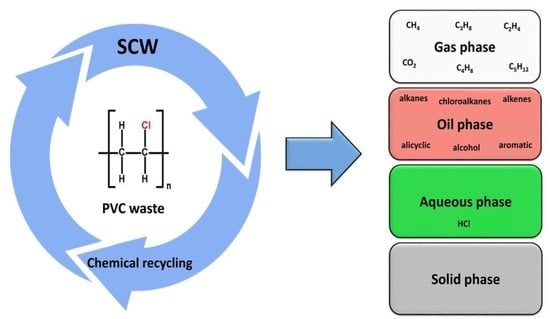
Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degradation of PVC Waste in SCW
2.3. Decomposition Products Analysis
2.3.1. FTIR Analysis of PVC Waste and Solid Residue
2.3.2. Analysis of the Oil and Gas Phase Composition after SCW Degradation
2.3.3. Determination of Chloride Ions in the Aqueous Phase
2.3.4. The Energetic Aspect of the Laboratory Process
3. Results and Discussion
3.1. The Yield of Degradation Products after Hydrothermal Decomposition of Colorless PVC Waste
3.2. Chemical Composition of the Gas Phase
3.3. Chemical Components in the Oil Phase
3.4. FTIR Analysis of Colorless PVC and Solid Residue after Hydrolysis
3.5. Degradation Mechanism of PVC Waste in SCW
3.6. Electricity Cost for Laboratory Scale Hydrothermal Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemeš, J.J.; Fan, Y.V.; Jiang, P. Plastics: Friends or Foes? The Circularity and Plastic Waste Footprint. Energy Sources Part Recovery Util. Environ. Eff. 2021, 43, 1549–1565. [Google Scholar] [CrossRef]
- Hahladakis, J.; Iacovidou, E.; Gerasimidou, S. Plastic Waste in a Circular Economy. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press/Elsevier: Cambridge, MA, USA, 2020; pp. 481–512. ISBN 978-0-12-817880-5. [Google Scholar]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID Pollution: Impact of COVID-19 Pandemic on Global Plastic Waste Footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef] [PubMed]
- Helmer Pedersen, T.; Conti, F. Improving the Circular Economy via Hydrothermal Processing of High-Density Waste Plastics. Waste Manag. 2017, 68, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pyrolysis & PyOil Purification Plants. Available online: https://www.apchemi.com (accessed on 16 September 2022).
- ReOil: OMV’s Circular Economy Pilot Project. Available online: https://www.omv.com/en/news/reoil-200-000-kg-of-plastic-waste-recycled-with-omv-s-circular-economy-pilot-project- (accessed on 15 September 2022).
- Kononov, S. Michelin Launches Construction of Its First Tire Pyrolysis Plant | Weibold–Tire Recycling & Pyrolysis Consulting. Available online: https://www.michelin.com/en/press-releases/michelin-launches-construction-on-its-first-tire-recycling-plant-in-the-world/ (accessed on 15 September 2022).
- Recycled Plastic: Important Things to Know. Available online: https://theaseanpost.com/article/recycled-plastic-important-things-know (accessed on 15 September 2022).
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal Reactions of Agricultural and Food Processing Wastes in Sub- and Supercritical Water: A Review of Fundamentals, Mechanisms, and State of Research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, P.; Čolnik, M.; Finšgar, M.; Knez, Ž.; Škerget, M. Determination of C1C6 Hydrocarbons in Gaseous Plastic Degradation Products by GC–MS Method. Polym. Degrad. Stab. 2020, 182, 109386. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Wang, Q.; Zou, J.; Zhang, H.; Jin, H.; Li, X. Experimental Investigation on Gasification Characteristics of Plastic Wastes in Supercritical Water. Renew. Energy 2019, 135, 32–40. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Meng, X.; Liu, C.; Zhang, H.; Zhang, W.; Jin, H. Experimental Investigation on Gasification Characteristics of Polycarbonate (PC) Microplastics in Supercritical Water. J. Energy Inst. 2020, 93, 624–633. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Catalytic Supercritical Water Gasification of Plastics with Supported RuO2: A Potential Solution to Hydrocarbons–Water Pollution Problem. Process Saf. Environ. Prot. 2016, 102, 140–149. [Google Scholar] [CrossRef]
- Özdenkçi, K.; Prestipino, M.; Björklund-Sänkiaho, M.; Galvagno, A.; De Blasio, C. Alternative Energy Valorization Routes of Black Liquor by Stepwise Supercritical Water Gasification: Effect of Process Parameters on Hydrogen Yield and Energy Efficiency. Renew. Sustain. Energy Rev. 2020, 134, 110146. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of Operational Issues in Hydrothermal Liquefaction and Supercritical Water Gasification Processes: A Review. Biomass Convers. Biorefinery 2021, 1–28. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Hydrothermal Decomposition of Polyethylene Waste to Hydrocarbons Rich Oil. J. Supercrit. Fluids 2021, 169, 105136. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Kilaz, G.; Chen, W.-T.; Wang, N.-H.L. Conversion of Polyethylene Waste into Clean Fuels and Waxes via Hydrothermal Processing (HTP). Fuel 2020, 273, 117726. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Hrnčič, M.K.; Ravber, M.; Škerget, M. High Pressure Water Reforming of Biomass for Energy and Chemicals: A Short Review. J. Supercrit. Fluids 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Knez, Ž.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and Value Added Compounds from Biomass Using Sub- and Supercritical Water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Adschiri, T. Chapter 4—Biomass Conversion in Supercritical Water. In Supercritical Fluid Technology for Energy and Environmental Applications; Anikeev, V., Fan, M., Eds.; Elsevier: Boston, MA, USA, 2014; pp. 89–98. ISBN 978-0-444-62696-7. [Google Scholar]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental Investigation on Liquefaction of Plastic Waste to Oil in Supercritical Water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef]
- Gemechu, E.D.; Kumar, A. Chapter 12—The Environmental Performance of Hydrogen Production Pathways Based on Renewable Sources. In Renewable-Energy-Driven Future; Ren, J., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 375–406. ISBN 978-0-12-820539-6. [Google Scholar]
- Sawai, O.; Nunoura, T.; Yamamoto, K. Supercritical Water Gasification of Sewage Sludge Using Bench-Scale Batch Reactor: Advantages and Drawbacks. J. Mater. Cycles Waste Manag. 2014, 16, 82–92. [Google Scholar] [CrossRef]
- Cao, C.; Bian, C.; Wang, G.; Bai, B.; Xie, Y.; Jin, H. Co-Gasification of Plastic Wastes and Soda Lignin in Supercritical Water. Chem. Eng. J. 2020, 388, 124277. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic Hydrothermal Gasification of Biomass. Biofuels Bioprod. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Akovali, G. 2—Plastic Materials: Polyvinyl Chloride (PVC). In Toxicity of Building Materials; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 23–53. ISBN 978-0-85709-122-2. [Google Scholar]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal Degradation of PVC: A Review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC Wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. [Google Scholar] [CrossRef]
- Takeshita, Y.; Kato, K.; Takahashi, K.; Sato, Y.; Nishi, S. Basic Study on Treatment of Waste Polyvinyl Chloride Plastics by Hydrothermal Decomposition in Subcritical and Supercritical Regions. J. Supercrit. Fluids 2004, 31, 185–193. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A. Photostabilization of Poly(Vinyl Chloride)—Still on the Run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High Efficiency Chlorine Removal from Polyvinyl Chloride (PVC) Pyrolysis with a Gas-Liquid Fluidized Bed Reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-H.; Jung, S.-H.; Kim, J.-S. Pyrolysis of Mixed Plastic Wastes for the Recovery of Benzene, Toluene, and Xylene (BTX) Aromatics in a Fluidized Bed and Chlorine Removal by Applying Various Additives. Energy Fuels 2010, 24, 1389–1395. [Google Scholar] [CrossRef]
- Borgianni, C.; De Filippis, P.; Pochetti, F.; Paolucci, M. Gasification Process of Wastes Containing PVC. Fuel 2002, 81, 1827–1833. [Google Scholar] [CrossRef]
- McNeill, I.C.; Memetea, L.; Cole, W.J. A Study of the Products of PVC Thermal Degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Xu, Z.; Kolapkar, S.S.; Zinchik, S.; Bar-Ziv, E.; McDonald, A.G. Comprehensive Kinetic Study of Thermal Degradation of Polyvinylchloride (PVC). Polym. Degrad. Stab. 2020, 176, 109148. [Google Scholar] [CrossRef]
- Keane, M.A. Catalytic Conversion of Waste Plastics: Focus on Waste PVC. J. Chem. Technol. Biotechnol. 2007, 82, 787–795. [Google Scholar] [CrossRef]
- Keane, M.A. Catalytic Transformation of Waste Polymers to Fuel Oil. ChemSusChem 2009, 2, 207–214. [Google Scholar] [CrossRef]
- Ali, M.F.; Siddiqui, M.N. Thermal and Catalytic Decomposition Behavior of PVC Mixed Plastic Waste with Petroleum Residue. J. Anal. Appl. Pyrolysis 2005, 74, 282–289. [Google Scholar] [CrossRef]
- Endo, K.; Emori, N. Dechlorination of Poly(Vinyl Chloride) without Anomalous Units under High Pressure and at High Temperature in Water. Polym. Degrad. Stab. 2001, 74, 113–117. [Google Scholar] [CrossRef]
- Shin, S.-M.; Yoshioka, T.; Okuwaki, A. Dehydrochlorination Behavior of Flexible PVC Pellets in NaOH Solutions at Elevated Temperature. J. Appl. Polym. Sci. 1998, 67, 2171–2177. [Google Scholar] [CrossRef]
- Yoshinaga, T.; Yamaye, M.; Kito, T.; Ichiki, T.; Ogata, M.; Chen, J.; Fujino, H.; Tanimura, T.; Yamanobe, T. Alkaline Dechlorination of Poly(Vinyl Chloride) in Organic Solvents under Mild Conditions. Polym. Degrad. Stab. 2004, 86, 541–547. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Imai, S.; Noritsune, M.; Okuwaki, A. Dechlorination of Poly(Vinylidene Chloride) in NaOH/Ethylene Glycol as a Function of NaOH Concentration, Temperature, and Solvent. Polym. Degrad. Stab. 2008, 93, 1979–1984. [Google Scholar] [CrossRef]
- Kershaw, J.; Edmond, L.; Rizzardo, E. Diels-Alder Reactions of Partially Dehydrochlorinated PVC in Solution and in the Melt. Polymer 1989, 30, 360–363. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Liu, Y.; Zhang, R. Review of Chloride Ion Detection Technology in Water. Appl. Sci. 2021, 11, 11137. [Google Scholar] [CrossRef]
- Gandon-Ros, G.; Soler, A.; Aracil, I.; Gómez-Rico, M.F. Dechlorination of Polyvinyl Chloride Electric Wires by Hydrothermal Treatment Using K2CO3 in Subcritical Water. Waste Manag. 2020, 102, 204–211. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Angyal, A. Pyrolysis of Polyvinyl Chloride (PVC)-Containing Mixed Plastic Wastes for Recovery of Hydrocarbons. Energy Fuels 2009, 23, 2743–2749. [Google Scholar] [CrossRef]
- Vansant, J.; Koziel, P.-W. Technical and Industrial Applications of CO2. In An Economy Based on Carbon Dioxide and Water: Potential of Large Scale Carbon Dioxide Utilization; Aresta, M., Karimi, I., Kawi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–103. ISBN 978-3-030-15868-2. [Google Scholar]
- Sato, Y.; Kato, K.; Takeshita, Y.; Takahashi, K.; Nishi, S. Decomposition of Ployvinylchloride Using Supercritical Water. Jpn. J. Appl. Phys. 1998, 37, 6270. [Google Scholar] [CrossRef]
- Lois, E.; Keating, E.L.; Gupta, A.K. Fuels. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 275–314. ISBN 978-0-12-227410-7. [Google Scholar]
- Casazza, A.; Spennati, E.; Converti, A.; Busca, G. Study on the Thermal Decomposition of Plastic Residues. Chem. Eng. Trans. 2019, 74, 1141–1146. [Google Scholar] [CrossRef]
- Baum, B.; Wartman, L.H. Structure and Mechanism of Dehydrochlorination of Polyvinyl Chloride. J. Polym. Sci. 1958, 28, 537–546. [Google Scholar] [CrossRef]
- HydroPRSTM. Available online: https://muratechnology.com/hydroprs/ (accessed on 16 September 2022).
- Hydrochloric Acid—Rolled Alloys, Inc. Available online: https://www.rolledalloys.com/technical-resources/environments/hydrochloric-acid/ (accessed on 15 September 2022).
- C-276 Hastelloy Alloy Coil-Tisco Shanxi Stainless Steel Co., Ltd. Available online: https://www.tiscoalloy.com/products/hastelloy/hastelloy_alloy_coil/242750.html (accessed on 15 September 2022).




| Components | 400 °C, 30 min Peak Area (%) | 400 °C, 60 min Peak Area (%) | 425 °C, 60 min Peak Area (%) |
|---|---|---|---|
| Hydrogen | 2.82 ± 0.08 | 1.00 ± 0.01 | 0.82 ± 0.01 |
| CO2 | 40.52 ± 1.10 | 36.71 ± 0.78 | 17.93 ± 0.45 |
| Methane | 2.24 ± 0.06 | 3.00 ± 0.01 | 3.10 ± 0.05 |
| Ethene | 1.50 ± 0.04 | 1.50 ± 0.02 | 0.52 ± 0.01 |
| Ethane | 5.80 ± 0.12 | 5.40 ± 0.13 | 10.71 ± 0.28 |
| Propene | 5.01 ± 0.12 | 3.10 ± 0.06 | 1.01 ± 0.01 |
| Propane | 7.43 ± 0.11 | 6.60 ± 0.17 | 17.41 ± 0.43 |
| Isobutane | 5.42 ± 0.16 | 1.10 ± 0.01 | 14.94 ± 0.21 |
| 2-Methyl-1-propene | 4.41 ± 0.07 | 6.02 ± 0.12 | 1.40 ± 0.02 |
| Butane | 5.60 ± 0.12 | 5.60 ± 0.07 | 11.21 ± 0.22 |
| 2-Butene | 7.10 ± 0.18 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2-Methylbutane | 0.90 ± 0.02 | 1.10 ± 0.01 | 8.40 ± 0.09 |
| 2-Pentene | 2.50 ± 0.01 | 1.10 ± 0.02 | 0.221 ± 0.006 |
| Pentane | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.21 ± 0.06 |
| 1-Methylcyclopentene | 0.0 ± 0.0 | 12.52 ± 0.21 | 5.3 ± 0.11 |
| Benzene | 2.40 ± 0.05 | 6.54 ± 0.06 | 1.72 ± 0.04 |
| Main Groups | Most Represented Components (Peak Area (%)) |
|---|---|
| Saturated aliphatic hydrocarbons (24.41 ± 0.51%) | Octanes (methyl, ethyl, dimethyl) (5.3 ± 0.09%) Decane (2.1 ± 0.03%) Tridecane (4.2 ± 0.11%) Pentadecane (6.04 ± 0.05%) Hexadecane (1.3 ± 0.01%) Octadecane (1.7 ± 0.03%) Heneicosane (1.2 ± 0.01%) |
| Halogenated hydrocarbons (chloroalkanes) (15.33 ± 0.38%) | 1-chloro-nonane (4.8 ± 0.12%) 1-chloro-decane (4.9 ± 0.09%) 1-chloro-undecane (5.4 ± 0.15%) |
| Unsaturated aliphatic hydrocarbons (3.80 ± 0.11%) | 3,3,5-trimethyl-1-hexene (1.8 ± 0.04%) 2,4-dimethyl-1-heptene (1.9 ± 0.01%) |
| Alicyclic hydrocarbons (0.609 ± 0.013%) | Cyclopentane (0.32 ± 0.011%) |
| Aromatic hydrocarbons (39.31 ± 0.93%) | Benzene derivatives (18.7 ± 0.39%) Naphthalene derivatives (12.4 ± 0.19%) Polycyclic aromatic hydrocarbons (2.6 ± 0.06%) |
| Alcohols (5.23 ± 0.12%) | 2-methyl-1-butanol (0.7 ± 0.012%) 6-methyl-1-heptanol (1.1 ± 0.014%) 3,7-dimethyl1-octanol (1.2 ± 0.02%) 1-nonanol (1.1 ± 0.019%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes 2022, 10, 1940. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10101940
Čolnik M, Kotnik P, Knez Ž, Škerget M. Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water. Processes. 2022; 10(10):1940. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10101940
Chicago/Turabian StyleČolnik, Maja, Petra Kotnik, Željko Knez, and Mojca Škerget. 2022. "Degradation of Polyvinyl Chloride (PVC) Waste with Supercritical Water" Processes 10, no. 10: 1940. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10101940