Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load
Abstract
:1. Introduction
2. Materials and Methods
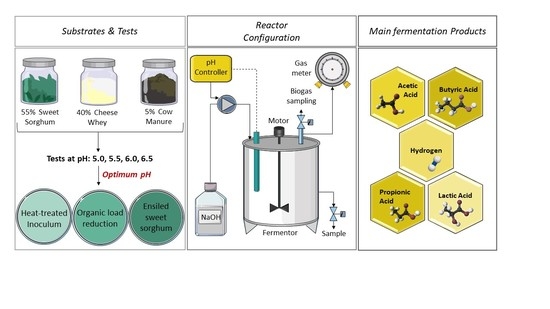
2.1. Substrates and Inoculum
2.2. Experimental Conditions
2.3. Analytical Methods
3. Results and Discussion
3.1. Chemical Composition of Each Substrate
3.2. Effect of pH
3.3. Kinetic Analysis
3.4. Heat-Treated Inoculum
3.5. Initial Organic Load
3.6. Effect of Ensiled Sorghum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, A.; Martinho, G. Waste hierarchy index for circular economy in waste management. Waste Manag. 2019, 95, 298–305. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Razo-Flores, E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: Influence of initial substrate concentration and pH. Int. J. Hydrogen Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Hu, S.; Wu, L.; Persson, S.; Peng, L.; Feng, S. Sweet sorghum and Miscanthus: Two potential dedicated bioenergy crops in China. J. Integr. Agric. 2017, 16, 1236–1243. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, D. A review of sweet sorghum as a viable renewable bioenergy crop and its techno-economic analysis. Renew. Energy 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Evaluation of dried sweet sorghum stalks as raw material for methane production. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Głąb, L.; Sowiński, J.; Chmielewska, J.; Prask, H.; Fugol, M.; Szlachta, J. Comparison of the energy efficiency of methane and ethanol production from sweet sorghum (Sorghum bicolor (L.) Moench) with a variety of feedstock management technologies. Biomass Bioenergy 2019, 129, 105332. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; de Vrije, T.; Budde, M.A.W.; Koukios, E.G.; Gylnos, A.; Reczey, K. Biological hydrogen production from sweet sorghum by thermophilic bacteria. In Proceedings of the 2nd World Conference on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004; pp. 1522–1525. [Google Scholar]
- Ntaikou, I.; Gavala, H.N.; Kornaros, M.; Lyberatos, G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int. J. Hydrogen Energy 2008, 33, 1153–1163. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.A.; Bakker, R.R.; De Vrije, T.; Koukios, E.G.; Claassen, P.A.M. Pretreatment of sweet sorghum bagasse for hydrogen production by Caldicellulosiruptor saccharolyticus. Int. J. Hydrogen Energy 2010, 35, 7738–7747. [Google Scholar] [CrossRef]
- Miaoyin, D.; Qiaoqiao, L.; Xu, F.; Wang, S.; Chen, J.; Li, W. Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet sorghum bagasse silage. Sci. Rep. 2020, 10, 837. [Google Scholar]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef]
- Yu, N.; Tan, L.; Sun, Z.-Y.; Nishimura, H.; Takei, S.; Tang, Y.-Q.; Kida, K. Bioethanol from sugarcane bagasse: Focused on optimum of lignin content and reduction of enzyme addition. Waste Manag. 2018, 76, 404–413. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag. 2017, 69, 407–422. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 2009, 248, 891–906. [Google Scholar] [CrossRef]
- Giuliano, A.; Bolzonella, D.; Pavan, P.; Cavinato, C.; Cecchi, F. Co-digestion of livestock effluents, energy crops and agro-waste: Feeding and process optimization in mesophilic and thermophilic conditions. Bioresour. Technol. 2013, 128, 612–618. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2020, 147. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.J. Modeling and optimization of anaerobic digested sludge converting starch to hydrogen. Biotechnol. Bioeng. 2000, 68, 269–278. [Google Scholar] [CrossRef]
- Ginkel, S.; Van Sung, S.; Lay, J.-J. Biohydrogen Production as a Function of pH and Substrate Concentration. Environ. Sci. Technol. 2001, 35, 4726–4730. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.; Gottschalk, G. Biochemistry and regulation of acid and solvent production in clostridia. In The Clostridia and Biotechnology; Woods, D.R., Ed.; Butterworth-Heinemann: Stoneham, MA, USA, 1993; pp. 25–50. [Google Scholar]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, W.; Gao, X.; Zhou, Y.; Shen, R. Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Manag. 2012, 32, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Dong, C.; Miller, C.; Li, Y.; Wang, L.; Yao, W. Continuous biohydrogen production from liquid swine manure supplemented with glucose using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2009, 34, 6636–6645. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark fermentation of complex waste biomass for biohydrogen production by pretreated thermophilic anaerobic digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Penniston, J.; Gueguim Kana, E.B. Impact of medium pH regulation on biohydrogen production in dark fermentation process using suspended and immobilized microbial cells. Biotechnol. Biotechnol. Equip. 2018, 32, 204–212. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater. In Standard Methods, 22nd ed.; Byrd Prepess: Springfield, WA, USA, 2012; ISBN 9780875532356. [Google Scholar]
- Joseffson, B. Rapid spectrophotometric determination of total carbohydrates. In Methods of Seawater Analysis; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie International: Weinheim, Germany; Deerfield Beach, FL, USA; Basel, Switzerland, 1983; pp. 340–342. ISBN 3527259988. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 analytical procedure—Determination of structural carbohydrates and lignin in Biomass. Lab. Anal. Proced. 2008, 1, 1–16. [Google Scholar]
- Vavouraki, A.I.; Volioti, V.; Kornaros, M.E. Optimization of thermo-chemical pretreatment and enzymatic hydrolysis of kitchen wastes. Waste Manag. 2014, 34, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, A.P.; Hernández-García, H.; Cocotle-Ronzón, Y.; Hernandez-Martinez, E. Methanogenesis of raw cheese whey: pH and substrate–inoculum ratio evaluation at mesophyll temperature range. J. Chem. Technol. Biotechnol. 2020, 95, 1946–1952. [Google Scholar] [CrossRef]
- Gallagher, D.; Parker, D.; Allen, D.J.; Tsesmetzis, N. Dynamic bacterial and fungal microbiomes during sweet sorghum ensiling impact bioethanol production. Bioresour. Technol. 2018, 264, 163–173. [Google Scholar] [CrossRef]
- Castelló, E.; Ferraz-Junior, A.D.N.; Andreani, C.; del Pilar Anzola-Rojas, M.; Borzacconi, L.; Buitrón, G.; Carrillo-Reyes, J.; Gomes, S.D.; Maintinguer, S.I.; Moreno-Andrade, I. Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions. Renew. Sustain. Energy Rev. 2020, 119, 109602. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2019, 11, 539–551. [Google Scholar] [CrossRef]
- Hernández, M.; Rodríguez, M. Hydrogen production by anaerobic digestion of pig manure: Effect of operating conditions. Renew. Energy 2013, 53, 187–192. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Zakoura, M.V.; Dareioti, M.A.; Kornaros, M. Biodegradation of Polyphenolic Compounds from Olive Mill Wastewaters (OMW) during Two-stage anaerobic Co-digestion of Agro-industrial mixtures. Waste Biomass Valorization 2019, 11, 5783–5791. [Google Scholar] [CrossRef]
- Zagklis, D.; Papadionysiou, M.; Tsigkou, K.; Tsafrakidou, P.; Zafiri, C.; Kornaros, M. Effect of pH on the Economic Potential of Dark Fermentation Products from Used Disposable Nappies and Expired Food Products. Appl. Sci. 2021, 11, 4099. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Angelopoulos, K.; Lyberatos, G. Biofuels generation from sweet sorghum: Fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour. Technol. 2008, 99, 110–119. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R. Effect of pH value on biohydrogen production from cheese whey. In Proceedings of the 4th International Symposium on Energy from Biomass and Waste, Venice, Italy, 12–15 November 2012; pp. 1–12. [Google Scholar]
- Bhat, J.V.; Barker, H.A. Clostridium lacto-acetophilum Nov. Spec. and the Role of Acetic Acid in the Butyric Acid Fermentation of Lactate. J. Bacteriol. 1947, 54, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Kumar, A.; Jianzheng, J.; Li, Y.; Baral, Y.N.; Ai, B. A review on bio-butyric acid production and its optimization. Int. J. Agric. Biol. 2014, 16, 1019–1024. [Google Scholar]
- Dessì, P.; Porca, E.; Frunzo, L.; Lakaniemi, A.; Collins, G.; Esposito, G.; Lens, P.N.L. Inoculum pretreatment differentially affects the active microbial community performing mesophilic and thermophilic dark fermentation of xylose. Int. J. Hydrogen Energy 2018, 43, 9233–9245. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Kucharska, K.; Gębicki, J. Mesophilic and thermophilic dark fermentation course analysis using sensor matrices and chromatographic techniques. Chem. Pap. 2020, 74, 1573–1582. [Google Scholar] [CrossRef]
- Slezak, R.; Grzelak, J.; Krzystek, L.; Ledakowicz, S. The effect of initial organic load of the kitchen waste on the production of VFA and H2 in dark fermentation. Waste Manag. 2017, 68, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Rangela, C.; Sastoqueb, J.; Calderonb, J.; Mosquerab, J.; Velasquezd, P.; Cabezabc, I.; Acevedobd, P. Hydrogen Production by Dark Fermentation Process: Effect of Initial Organic Load. Chem. Eng. 2020, 79. [Google Scholar] [CrossRef]






| Parameter | Units | CW | LCM |
|---|---|---|---|
| pH | - | 6.17 ± 0.03 | 7.26 ± 0.18 |
| TSS | g/L | 38.00 ± 0.11 | 48.60 ± 0.24 |
| VSS | g/L | 34.60 ± 0.14 | 35.80 ± 0.53 |
| TS | g/L | 69.40 ± 0.34 | 52.40 ± 0.93 |
| VS | g/L | 60.17 ± 0.25 | 35.83 ± 0.64 |
| Total COD | g/L | 75.00 ± 0.29 | 62.50 ± 2.12 |
| Soluble COD | g/L | 66.73 ± 0.17 | 23.02 ± 0.26 |
| TOC | g/L | 31.45 ± 0.64 | 24.19 ± 0.16 |
| Total carbohydrates 1 | g/L | 64.40 ± 0.90 | 8.12 ± 0.31 |
| Soluble carbohydrates 1 | g/L | 62.80 ± 1.04 | 1.20 ± 0.01 |
| TKN | g/L | 0.84 ± 0.08 | 3.36 ± 0.00 |
| Ammonium-N | g/L | 0.10 ± 0.02 | 2.39 ± 0.02 |
| Alkalinity | g CaCO3/L | 0.50 ± 0.00 | 12.38 ± 0.32 |
| TVFA | g/L | 0.07 ± 0.00 | 7.24 ± 0.02 |
| Parameter | FS | ES |
|---|---|---|
| pH | 5.6 ± 0.1 | 4.6 ± 0.2 |
| Moisture (% wet weight) | 76.7 ± 0.1 | 77.7 ± 0.3 |
| TS (% wet weight) | 23.3 ± 1.0 | 22.3 ± 0.2 |
| VS (%DM) | 94.5 ± 0.2 | 94.4 ± 0.1 |
| Ash (%DM) | 5.5 ± 0.3 | 5.6 ± 0.8 |
| TOC (%DM) | 59.8 ± 0.1 | 51.0 ± 1.3 |
| Total carbohydrates 1 (%DM) | 81.0 ± 3.1 | 36.9 ± 1.0 |
| Soluble carbohydrates 1 (%DM) | 49.0 ± 0.3 | 2.9 ± 0.1 |
| Cellulose (%DM) | 23.0 ± 0.4 | 27.9 ± 2.1 |
| Hemicellulose (%DM) | 11.0 ± 0.7 | 12.5 ± 1.2 |
| Lignin (%DM) | 8.0 ± 0.4 | 11.9 ± 3.1 |
| Total Nitrogen, TKN (%DM) | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Proteins (%DM) | 0.7 ± 0.1 | 1.1 ± 0.1 |
| Lactic Acid (%DM) | N.D. | 1.2 ± 0.1 |
| Ethanol (%DM) | N.D. | 8.9 ± 0.1 |
| Reactions for Period: 0–8.5 h | Equation | Calculated Values (mmoles) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glucose | Lactic | Ethanol | Butyric | Acetic | Propionic | H2 | ||
| 1. C6H12O6→2CH3CH(OH)COOH | (3) | −6.76 | +13.52 | |||||
| 2. C6H12O6→CH3CH(OH)COOH + CH3CH2OH + CO2 | (4) | −9.04 | +9.04 | +9.04 | ||||
| 3. 2C6H12O6→3CH3COOH + 2CH3CH(OH)COOH | (5) | −75.25 | +75.25 | +112.87 | ||||
| 4. 3CH3CH(OH)COOH→2CH3CH2COOH + CH3COOH + CO2 + H2O | (6) | −13.08 | +4.36 | +8.72 | ||||
| 5. 2CH3CH(OH)COOH→CH3CH2CH2COOH + 2CO2 + 2H2 | (7) | −16.40 | +8.20 | +16.40 | ||||
| 6. 2CH3COOH + 2H2→CH3CH2CH2COOH + 2H2O | (8) | +5.80 | −11.60 | −11.60 | ||||
| Net calculated values | −91.05 | +68.33 | +9.04 | +14.08 | +105.63 | +8.72 | +4.80 | |
| Experimental values | −91.05 | +64.17 | +9.04 | +14.08 | +105.63 | +8.72 | +4.78 | |
| Error (%) | 0.00 | 6.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.42 | |
| Reactions for period: 8.5–44.5 h | ||||||||
| 7. C6H12O6→2CH3CH(OH)COOH | (3) | −10.67 | +21.34 | |||||
| 8 C6H12O6→CH3CH(OH)COOH + CH3CH2OH + CO2 | (4) | −0.70 | +0.70 | +0.70 | ||||
| 9. 2C6H12O6→3CH3COOH + 2CH3CH(OH)COOH | (5) | −0.00 | +0.00 | +0.00 | ||||
| 10. 3CH3CH(OH)COOH→2CH3CH2COOH + CH3COOH + CO2 + H2O | (6) | −8.60 | +2.87 | +5.74 | ||||
| 11. 2CH3CH(OH)COOH→CH3CH2CH2COOH + 2CO2 + 2H2 | (7) | −69.18 | +34.59 | +69.18 | ||||
| 12. 2CH3COOH + 2H2→CH3CH2CH2COOH + 2H2O | (8) | +10.95 | −21.90 | −21.90 | ||||
| Net calculated values | −11.37 | −55.74 | +0.70 | +45.54 | −19.03 | +5.74 | +47.28 | |
| Experimental values | −11.37 | −55.74 | +0.70 | +45.54 | −19.80 | +5.74 | +47.55 | |
| Error (%) | 0.00 | 0.00 | 0.00 | 0.00 | 3.89 | 0.00 | 0.57 | |
| Reactions for Period: 0–8.5 h | Equation | Calculated Values (mmoles) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glucose | Lactic | Ethanol | Butyric | Acetic | Propionic | H2 | ||
| 1. C6H12O6→2CH3CH(OH)COOH | (3) | −42.02 | +84.04 | |||||
| 2. C6H12O6→CH3CH(OH)COOH + CH3CH2OH + CO2 | (4) | −0.96 | +0.96 | +0.96 | ||||
| 3. 2C6H12O6→3CH3COOH + 2CH3CH(OH)COOH | (5) | −8.96 | +8.96 | +13.44 | ||||
| 4. 3CH3CH(OH)COOH→2CH3CH2COOH + CH3COOH + CO2 + H2O | (6) | −0.00 | +0.00 | +0.00 | ||||
| 5. 2CH3CH(OH)COOH→CH3CH2CH2COOH + 2CO2 + 2H2 | (7) | −0.00 | +0.00 | +0.00 | ||||
| 6. 2CH3COOH+2H2→CH3CH2CH2COOH + 2H2O | (8) | +0.00 | −0.00 | −0.00 | ||||
| Net calculated values | −51.94 | +93.96 | +0.96 | +0.00 | +13.44 | +0.00 | +0.00 | |
| Experimental values | −51.94 | +92.35 | +0.96 | +0.00 | +13.44 | +0.00 | +0.00 | |
| Error (%) | 0.00 | 1.74 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Reactions for period: 8.5–44.5 h | ||||||||
| 7. C6H12O6→2CH3CH(OH)COOH | (3) | −9.31 | +18.62 | |||||
| 8 C6H12O6→CH3CH(OH)COOH + CH3CH2OH + CO2 | (4) | −0.00 | +0.00 | +0.00 | ||||
| 9. 2C6H12O6→3CH3COOH + 2CH3CH(OH)COOH | (5) | −1.94 | +1.94 | +2.91 | ||||
| 10. 3CH3CH(OH)COOH→2CH3CH2COOH + CH3COOH + CO2 + H2O | (6) | −13.46 | +4.49 | +8.97 | ||||
| 11. 2CH3CH(OH)COOH→CH3CH2CH2COOH + 2CO2 + 2H2 | (7) | −85.46 | +42.73 | +85.46 | ||||
| 12. 2CH3COOH+2H2→CH3CH2CH2COOH + 2H2O | (8) | +10.21 | −20.42 | −20.42 | ||||
| Net calculated values | −11.25 | −78.36 | +0.00 | +52.94 | −13.02 | +8.97 | +65.04 | |
| Experimental values | −11.25 | −78.36 | +0.00 | +52.94 | −13.76 | +8.97 | +60.12 | |
| Error (%) | 0.00 | 0.00 | 0.00 | 0.00 | 5.38 | 0.00 | 8.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load. Processes 2021, 9, 1017. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061017
Dareioti MA, Vavouraki AI, Tsigkou K, Zafiri C, Kornaros M. Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load. Processes. 2021; 9(6):1017. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061017
Chicago/Turabian StyleDareioti, Margarita Andreas, Aikaterini Ioannis Vavouraki, Konstantina Tsigkou, Constantina Zafiri, and Michael Kornaros. 2021. "Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load" Processes 9, no. 6: 1017. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061017