Insidious-Onset Indurated Plaques on the Shins
Abstract
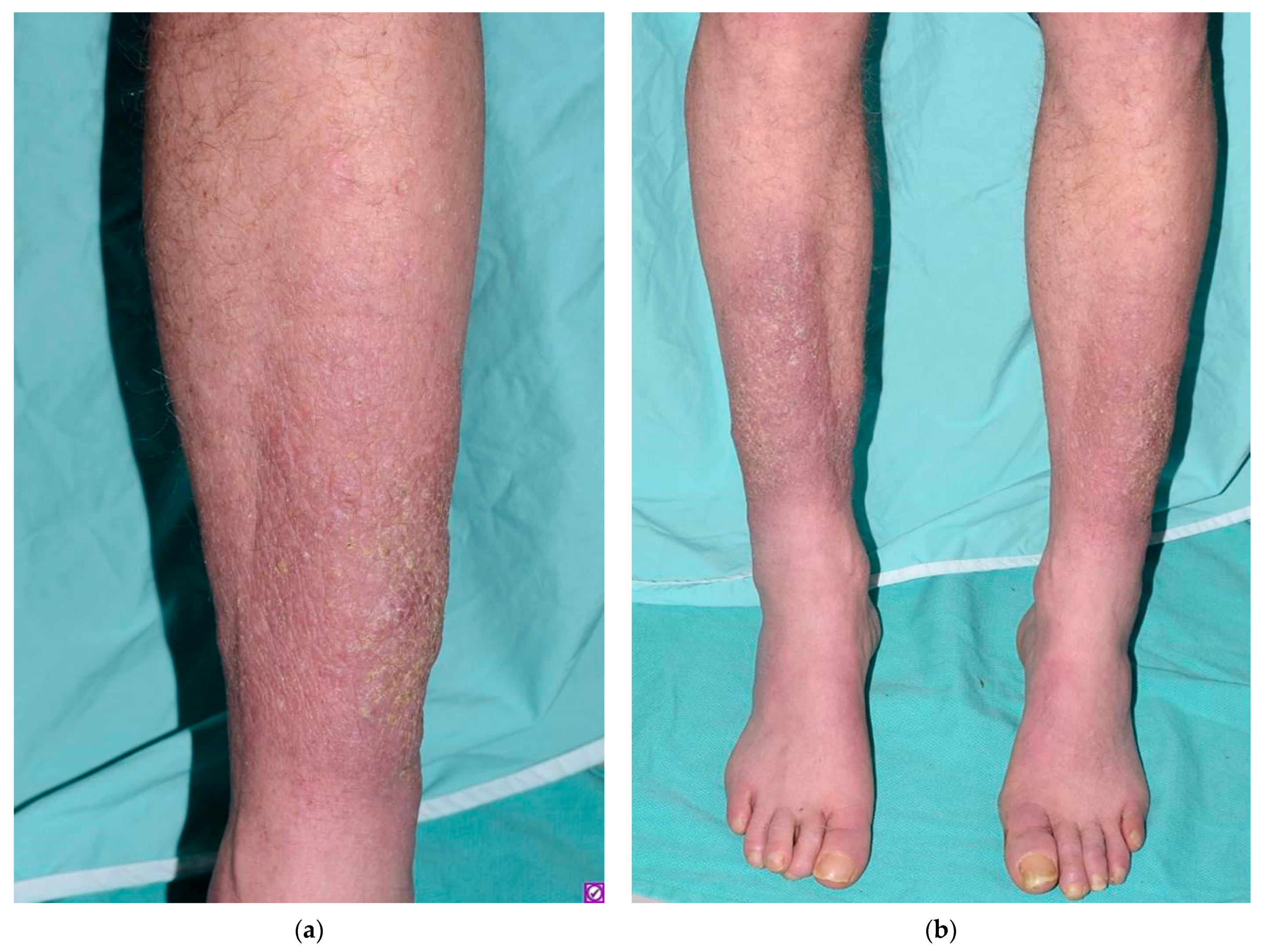
:1. Introduction
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fatourechi, V. Pretibial myxedema: Pathophysiology and treatment options. Am. J. Clin. Dermatol. 2005, 6, 295–309. [Google Scholar] [CrossRef]
- Bahn, R.S. Clinical review 157: Pathophysiology of Graves’ ophthalmopathy: The cycle of disease. J. Clin. Endocrinol. Metab. 2003, 88, 1939–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayasi, T.; Danielsen, L.; Asboe-Hansen, G. Ultrastructure of localized myxedema. Acta Derm. Venereol. 1976, 56, 173–185. [Google Scholar] [PubMed]
- Ishii, M.; Nakagawa, K.; Hamada, T. An ultrastructural study of pretibial myxedema utilizing improved ruthenium red stain. J. Cutan. Pathol. 1984, 11, 125–131. [Google Scholar] [CrossRef]
- Bartalena, L.; Bogazzi, F.; Tanda, M.L.; Manetti, L.; Dell’Unto, E.; Martino, E. Cigarette smoking and the thyroid. Eur. J. Endocrinol. 1995, 133, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, J.B.; Hegedüs, L. Cigarette Smoking and the Thyroid. Thyroid 1994, 4, 327–331. [Google Scholar] [CrossRef]
- Kriss, J.P.; Pleshakov, V.; Rosenblum, A.; Sharp, G. Therapy with Occlusive Dressings of Pretibial Myxedema with Fluocinolone Acetonide1. J. Clin. Endocrinol. Metab. 1967, 27, 595–604. [Google Scholar] [CrossRef]
- Schwartz, K.M.; Fatourechi, V.; Ahmed, D.D.F.; Pond, G.R. Dermopathy of Graves’ Disease (Pretibial Myxedema): Long-Term Outcome. J. Clin. Endocrinol. Metab. 2002, 87, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Volden, G. Successful treatment of chronic skin diseases with clobetasol propionate and a hydrocolloid occlusive dressing. Acta Derm. Venereol. 1992, 72, 69–71. [Google Scholar]
- Pineda, A.M.M.; Tianco, E.A.V.; Tan, J.B.; Casintahan, F.A.; Beloso, M.B. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J. Eur. Acad. Dermatol. Venereol. JEADV Engl. 2007, 21, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Engin, B.; Gümüşel, M.; Özdemir, M.; Çakir, M. Successful combined pentoxifylline and intralesional triamcino-lone acetonide treatment of severe pretibial myxedema. Dermatol. Online J. 2007, 13. Available online: https://escholarship.org/uc/item/8nd2c6tr (accessed on 3 June 2021).
- Kudlak, N.; Schuler, A.; Dong, J.; Crowe, D. Pretibial myxedema treated with intralesional hyaluronidase and triamcinolone. JAAD Case Rep. 2020, 6, 810. [Google Scholar] [CrossRef]
- Ramos, L.O.; Mattos, P.C.; Figueredo, G.L.P.; de Maia, A.A.A.; Romero, S.A.R. Pre-tibial myxedema: Treatment with intrale-sional corticosteroid. An. Bras. Dermatol. 2015, 90 (Suppl. 1), 143–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesly, P.M.; Tolaymat, L.M.; Sluzevich, J.C.; Keeling, J.H. Pretibial myxedema successfully treated with intralesional hy-aluronidase. JAAD Case Rep. 2018, 4, 874–876. [Google Scholar] [CrossRef]
- Jimenez, A.; Hull, C.; Zone, J. Rituximab therapy for a severe case of pretibial myxedema. JAAD Case Rep. 2021, 10, 28–30. [Google Scholar] [CrossRef]
- Kotwal, A.; Turcu, A.F.; Sonawane, V.; Bahn, R.S.; Pittelkow, M.R.; Bridges, A.; Stan, M.N. Clinical Experience with Rituximab and Intravenous Immunoglobulin for Pretibial Myxedema: A Case Series. Thyroid 2019, 29, 692–699. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Li, X.; Shi, R.; Zheng, J. Efficacy of Trimodality Therapy for Pretibial Myxoedema: A Case Series of 20 Patients. Acta Derm. Venereol. 2016, 96, 714–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.R.; Erickson, C.P.; Calame, A. Case report and review of solitary cutaneous focal mucinosis: A unique primary cutaneous mucinosis unrelated to mucinosis-associated systemic diseases. Dermatol Online J. 2020, 26, 4. [Google Scholar]
- Greene, A.K.; Goss, J.A. Diagnosis and Staging of Lymphedema. Semin Plast Surg. 2018, 32, 12–16. [Google Scholar]
- Olszewski, W.L.; Jamal, S.; Manokaran, G.; Lukomska, B.; Kubicka, U. Skin changes in filarial and non-filarial lymphoe-dema of the lower extremities. Trop. Med. Parasitol Off. Organ Dtsch Trop. Ges. Dtsch Ges. Tech. Zs. 1993, 44, 40–44. [Google Scholar]
- Rongioletti, F.; Donati, P.; Amantea, A.; Ferrara, G.; Montinari, M.; Santoro, F.; Parodi, A. Obesity-associated lymphoedematous mucinosis. J. Cutan. Pathol. 2009, 36, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Ferreli, C.; Pinna, A.L.; Pilloni, L.; Corbeddu, M.; Rongioletti, F. Obesity-Associated Lymphedematous Mucinosis: Two Further Cases and Review of the Literature. Dermatopathology 2018, 5, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Erfurt-Berge, C.; Seitz, A.-T.; Rehse, C.; Wollina, U.; Schwede, K.; Renner, R. Update on clinical and laboratory features in necrobiosis lipoidica: A retrospective multicentre study of 52 patients. Eur. J. Dermatol. EJD 2012, 22, 770–775. [Google Scholar] [CrossRef]
- Sibbald, C.; Reid, S.; Alavi, A. Necrobiosis Lipoidica. Dermatol. Clin. 2015, 33, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, B.T.; Mainetti, C.; Peeters, M.-A.; Laffitte, E. Cutaneous Granulomatosis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2018, 54, 131–146. [Google Scholar] [CrossRef]

| Lesion | Clinical Features | Histological Features |
|---|---|---|
| Pretibial myxedema | Seen in a minority of patients with Graves’ disease, more commonly affects females. Firm, non-pitting, scaly, thickened plaques or nodules are typically present on the shins or dorsum of feet. The skin may be slightly discolored and have a peau d’orange texture. Lesions can be pruritic and sore. | Extensive mucin deposition throughout the dermis and subcutis with collagen bundles widely separated or fragmented. Scattered dermal fibroblasts are often present without proliferation and there may be overlying epidermal hyperkeratosis and superficial perivascular lymphocytic infiltrate. |
| Focal cutaneous mucinosis (solitary) | Typically, an asymptomatic flesh-colored dome-shaped single papule smaller than 1 cm × 1 cm, most commonly found on the extremities. Most patients are between 29 and 60 years of age. It is slightly more common in males. In contrast, diffuse cutaneous mucinosis is associated with various systemic disease processes [18]. | Characteristic focal mucin deposition in the upper dermis. Can extend into the deeper dermis but rarely into the subcutaneous fat. There may be an increase in scattered fibroblasts and capillaries within the lesion. The epidermis may be atrophic or hyperplastic and dermal dendrocytes are passively incorporated into the lesion [18]. |
| Lymphedema | The primary disease results from malformation of lymphatic development and is rare. Secondary disease is acquired from damage to the lymphatic system. Parasitic filariasis is the most common global cause, although in developed countries, lymph node disruption, either surgical removal or irradiation, is the most common cause. There is a pitting edema in the affected limb with circumferential growth. Ulceration is not present, but there may be lymphatic vesicles and lymphorrhea. Skin can harden and thicken in the later stages [19]. | Filarial infection shows keratinocyte hyperproliferation, focal acantholysis, lymphocytic infiltrate at the dermo–epidermal junction, dermal perivascular mononuclear infiltrate and subepidermal granulocytic infiltrates. There may be ‘lymphatic lakes’ between thick collagen bundles. Immunohistochemistry shows abundant macrophages (CD68+) and positivity for HLA-DR in all mononuclear and endothelial cells. Non-filarial lymphedema shows moderate keratinocyte proliferation, increased numbers of epidermal Langerhans cells (CD1+), moderate perivascular lymphocytic infiltrate and much less cellular positivity for HLA-DR [20]. |
| Obesity-associated lymphedematous mucinosis | Can mimic pretibial myxedema but is classically associated with obesity; thyroid disorder is absent [21]. Typical skin-colored-to-yellowish papules, plaques and nodules arise in the pretibial region of lymphedematous legs [22]. | Four distinct features have been identified: epidermal atrophy, moderate mucin deposition in the superficial dermis, vertically running angioplasia in the mid and superficial dermis and an increase in fibroblasts [21]. |
| Necrobiosis lipoidica | Associated with type 1 diabetes mellitus, more commonly seen in females [23]. Characterized by enlarged firm red-brown papules that coalesce to form well-defined oval plaques with central yellowish-brown discoloration, atrophy and telangiectasias with a violaceous rim. Usually painless unless there is ulceration which may occur after trauma. Hypohidrosis and alopecia may develop within the plaque. Progression to squamous cell carcinoma has been reported [24]. | Layered granulomatous inflammatory process alternating between zones of necrobiosis running parallel to the skin surface involving the full thickness of the dermis and extending into the subcutaneous fat septae. Collagen is degenerated and the epidermis is typically normal or atrophic. Necrobiotic areas are poorly defined with an intervening inflammatory infiltrate predominantly lymphocytic with plasma cells. No significant mucin deposition in the center of the granulomas [25]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, R.; Knabel, D.; Fernandez, A.P. Insidious-Onset Indurated Plaques on the Shins. Dermatopathology 2021, 8, 185-189. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020024
Agrawal R, Knabel D, Fernandez AP. Insidious-Onset Indurated Plaques on the Shins. Dermatopathology. 2021; 8(2):185-189. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020024
Chicago/Turabian StyleAgrawal, Rishi, Daniel Knabel, and Anthony P. Fernandez. 2021. "Insidious-Onset Indurated Plaques on the Shins" Dermatopathology 8, no. 2: 185-189. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020024






