Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Stock and Calibration Standard Solutions
2.3. Sample Preparation Procedure
2.3.1. Cosmetic Formulation
2.3.2. Cosmetic Cream
2.4. HILIC-PDA
2.5. Method Validation and Application to the Analysis of Real Samples
3. Results and Discussions
3.1. HILIC-PDA Method Development
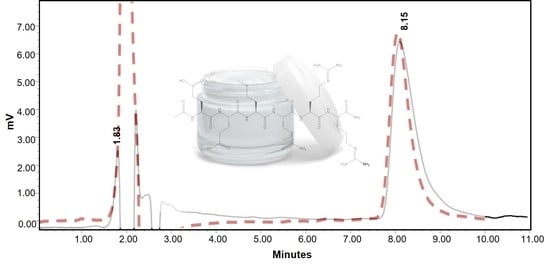
Chromatography
3.2. Statistical Analysis of Data
3.2.1. Selectivity
3.2.2. Linearity Data
3.2.3. Accuracy and Precision
3.3. Application to the Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lupo, M.P.; Cole, A.L. Cosmeceutical peptides. Derm. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef]
- Rivers, J.K. The role of cosmeceuticals in antiaging therapy. Skin Ther. Lett. 2008, 13, 5–9. [Google Scholar]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Derm. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Husein el Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Derm. 2016, 15, 514–519. [Google Scholar] [CrossRef]
- Sutton, R.B.; Fasshauer, D.; Jahr, R.; Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4. A resolution. Nature 1998, 395, 347–353. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Clemente, J.; Jodas, G.; Gil, A.; Fernández-Ballester, G.; Ponsati, B.; Gutierrez, L.; Pérez-Payá, E.; Ferrer-Montiel, A. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int. J. Cosmet. Sci. 2002, 24, 303–310. [Google Scholar] [CrossRef]
- Hoppel, M.; Reznicek, G.; Kahlig, H.; Kotisch, H.; Resch, G.P.; Valenta, C. Topical delivery of acetyl hexapeptide-8 from different emulsions: Influence of emulsion composition and internal structure. Eur. J. Pharm. Sci. 2015, 68, 27–35. [Google Scholar] [CrossRef]
- Blanes-Mira, C.; Merino, J.M.; Valera, E.; Fernández-Ballester, G.; Gutiérrez, L.M.; Viniegra, S.; Pérez-Payá, E.; Ferrer-Montiel, A. Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J. Neurochem. 2004, 88, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Grumelli, C.; Verderio, C.; Pozzi, D.; Rossetto, O.; Montecucco, C.; Matteoli, M. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology 2005, 26, 761–767. [Google Scholar] [CrossRef]
- Mira, M.C.B.; Llobregat Hernandez, M.M.; Tebar, A.I.G.; Fernandez Ballester, G.J.; Planell Cases, R.M.; Ferrer Montiel, A.V.; Viniegra Bover, S.; Gutierrez Perez, L.M.; Carbonell Castello, T.; Perez Paya, E. Neuronal Exocytosis Inhibiting Peptides and Cosmetic and Pharmaceutical Compositions Containing Sad Peptides. U.S. Patent 7,015,192 B1, 21 March 2006. Available online: https://patentimages.storage.googleapis.com/dc/2f/ec/3e6fef57fd6254/US7015192.pdf (accessed on 29 July 2021).
- Ruiz, M.A.; Clares, B.; Morales, M.E.; Cazalla, S.; Gallardo, V. Preparation and stability of cosmetic formulations with an anti-aging peptide. J. Cosmet. Sci. 2007, 58, 157–171. [Google Scholar] [CrossRef]
- Jankovic, J.; Kenney, C.; Grafe, S.; Goertelmeyer, R.; Comes, G. Relationship between various clinical outcome assessments in patients with blepharospasm. Mov. Disord. 2009, 24, 407–413. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xiao, X.S.; Huo, J.; Zhang, W.D. The anti-wrinkle efficacy of Argireline. J. Cosmet. Laser Ther. 2013, 15, 237–241. [Google Scholar] [CrossRef]
- Tandini, K.A.; Mercurio, D.G.; Gonçalves Maia Campos, P.M.B. Acetyl hexapeptide-3 in a cosmetic formulation acts on skin mechanical properties-clinical study. Braz. J. Pharm. Sci. 2015, 51, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Raikou, V.; Varvaresou, A.; Panderi, I.; Papageorgiou, E. The efficacy study of the combination of tripeptide-10 citrulline and acetyl hexapeptide-3. A prospective randomized controlled study. J. Cosmet. Dermatol. 2017, 16, 271–278. [Google Scholar] [CrossRef]
- Lungu, C.; Considine, E.; Zahir, S.; Ponsati, B.; Arrastiac, S.; Hallett, M. Pilot study of topical acetyl hexapeptide-8 in the treatment for blepharospasm in patients receiving botulinum toxin therapy. Eur. J. Neurol. 2013, 20, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T. Peptide separation by Hydrophilic-Interaction Chromatography: A review. J. Biochem. Biophys. Meth. 2004, 60, 265–280. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Kozlik, P.; Goldman, R.; Sanda, M. Hydrophilic interaction liquid chromatography in the separation of glycopeptides and their isomers. Anal. Bioanal. Chem. 2018, 410, 5001–5008. [Google Scholar] [CrossRef]
- Roca, L.S.; Schoemaker, S.E.; Pirok, B.W.J.; Gargano, A.F.G.; Schoenmakers, P.J. Accurate modelling of the retention behaviour of peptides in gradient-elution hydrophilic interaction liquid chromatography. J. Chromatogr. A 2020, 1614, 460650. [Google Scholar] [CrossRef]
- Molnarova, K.; Kozlík, P. Comparison of different HILIC stationary phases in the separation of hemopexin and immunoglobulin G glycopeptides and their isomers. Molecules 2020, 25, 4655. [Google Scholar] [CrossRef]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef]
- Fical, L.; Khalikova, M.; Kočová Vlčková, H.; Lhotská, I.; Hadysová, Z.; Vokřál, I.; Červený, L.; Švec, F.; Nováková, L. Determination of Antiviral Drugs and Their Metabolites Using Micro-Solid Phase Extraction and UHPLC-MS/MS in Reversed-Phase and Hydrophilic Interaction Chromatography Modes. Molecules 2021, 26, 2123. [Google Scholar] [CrossRef]
- Panderi, I.; Malamos, Y.; Machairas, G.; Zaharaki, S. Investigation of the retention mechanism of cephalosporins by zwitterionic hydrophilic interaction liquid chromatography. Chromatographia 2016, 79, 995–1002. [Google Scholar] [CrossRef]
- Panderi, I.; Taxiarchi, E.; Pistos, C.; Kalogria, E.; Vonaparti, A. Insights into the Mechanism of Separation of Bisphosphonates by Zwitterionic Hydrophilic Interaction Liquid Chromatography: Application to the Quantitation of Risedronate in Pharmaceuticals. Separations 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Dinh, N.P.; Jonsson, T.; Irgum, K. Water uptake on polar stationary phases under conditions for hydrophilic interaction chromatography and its relation to solute retention. J. Chromatogr. A 2013, 1320, 33–47. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T. Mobile phase effects on the retention on polar columns with special attention to the dual hydrophilic interaction–reversed-phase liquid chromatography mechanism, a review. J. Sep. Sci. 2018, 41, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Janviera, S.; De Sutter, E.; Wynendaele, E.; De Spiegeleer, B.; Vanhee, C.; Deconinck, E. Analysis of illegal peptide drugs via HILIC-DAD-MS. Talanta 2017, 174, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalmans, S.; Gevaert, B.; Verbeke, F.; D’Hondt, M.; Bracke, N.; Wynendaele, E.; De Spiegeleer, B. Quality control of cationic cell-penetrating peptides. J. Pharm. Biomed. Anal. 2016, 117, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chirita, R.I.; Chaimbault, P.; Archambault, J.C.; Robert, I.; Elfakir, C. Development of a LC–MS/MS method to monitor palmitoyl peptides content in anti-wrinkle cosmetics. Anal. Chim. Acta 2009, 641, 95–100. [Google Scholar] [CrossRef]
- Papagianni, P.; Varvaresou, A.; Papageorgiou, S.; Panderi, I. Development and validation of an ion-pair RP-HPLC method for the determination of oligopeptide-20 in cosmeceuticals. J. Pharm. Biomed. Anal. 2011, 56, 645–649. [Google Scholar] [CrossRef]
- Giannakou, M.; Varvaresou, A.; Kiriazopoulos, E.; Papageorgiou, S.; Kavvalou, E.; Tsirivas, E.; Panderi, I. Quantification of oligopeptide-20 and oligopeptide-24 in cosmetic creams using hydrophilic interaction liquid chromatography with electrospray ionization mass spectrometry. Sep. Sci. Plus 2018, 1, 159–167. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, P.G.; Krynitsky, A.J.; Rader, J.I. Rapid and simultaneous determination of hexapeptides (Ac-EEMQRR-amide and H2N-EEMQRR-amide) in anti-wrinkle cosmetics by hydrophilic interaction liquid chromatography–solid phase extraction preparation and hydrophilic interaction liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 7956–7963. [Google Scholar]
- Kraeling, M.E.K.; Zhou, W.; Wang, P.; Ogunsola, O.A. In vitro skin penetration of acetyl hexapeptide- 8 from a cosmetic formulation. J. Cutan. Ocular. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, P.G.; Ogunsola, O.A.; Kraeling, M.E.K. Rapid determination of hexapeptides by hydrophilic interaction LC-MS/MS for in vitro skin penetration studies. Bioanalysis 2013, 5, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- ICH. Guideline Q2(R1) Validation of Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonisation, London, UK 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 29 July 2021).
- Machairas, G.; Panderi, I.; Geballa-Koukoula, A.; Rozou, S.; Antonopoulos, N.; Charitos, C.; Vonaparti, A. Development and validation of a hydrophilic interaction liquid chromatography method for the quantitation of impurities in fixed-dose combination tablets containing rosuvastatin and metformin. Talanta 2018, 183, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, E.; Leknes, S.; Wilson, S.R.; Lundanes, E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCalley, D.V. Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2017, 1523, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, N.; Machairas, G.; Migias, G.; Vonaparti, A.; Brakoulia, V.; Pistos, C.; Gennimata, D.; Panderi, I. Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma. Molecules 2018, 23, 1548. [Google Scholar] [CrossRef] [Green Version]
- British Pharmacopoeia; Her Majesty’s Stationery Office: London, UK, 2004; Volume II, pp. 1428–1429.





| Concentration Range (μg mL−1) | 20–30 |
| Regression Equation | S = 18.08 (±0.64) × 103 C − 20 (±16) × 103 |
| Correlation Coefficient, R | 0.998 |
| Standard Error of Estimation, Sr | 5072 |
| Limit of Detection, LOD (μg mL−1) | 0.5 |
| Limit of Quantitation, LOQ (μg mL−1) | 1.5 |
| texperimental: a/Sa 1 | 1.24 |
| tp, f = 3, p = 0.05 2 | 4.30 |
| Concentration Range (% w/w) | 0.004–0.007 |
| Regression Equation | S = 1105 (±41) × C + 0.71 (±0.23) |
| Correlation Coefficient, r | 0.998 |
| Standard Error of Estimation, Sr | 0.098 |
| Limit of Detection, LOD (% w/w) | 6.8 × 10−4 |
| Limit of Quantitation, LOQ (% w/w) | 0.002 |
| texperimental: a/Sa 1 | 3.10 |
| tp, f = 3, p = 0.05 2 | 4.30 |
| Matrix | Concentration Levels | |||
|---|---|---|---|---|
| Cosmetic Formulation | ||||
| Added concentration (μg mL−1) | 20 | 25 | ||
| Overall mean | 19.95 (±0.91) | 24.74 (±0.42) | ||
| Intraday CV(%)1 | 4.76 | 1.66 | ||
| Total precision CV(%) 1 | 4.37 | 1.74 | ||
| Total accuracy Er%2 | 99.8 | 98.9 | ||
| Cosmetic cream | ||||
| Added concentration (% w/w) | 0.004 | 0.005 | 0.007 | |
| Overall mean | 39.7 (±0.82) × 10−4 | 50.62 (±0.39) × 10−4 | 69.71 (±0.79) × 10−4 | |
| Intraday, CV(%) 1 | 2.41 | 3.33 | 3.11 | |
| Total precision, CV(%) 1 | 2.46 | 3.35 | 3.53 | |
| Total accuracy, Relative Recovery (%) 2 | 99.3 | 101.2 | 99.6 | |
| Cosmetic Product | % Label Claim (±SD) 1 (n = 5) | % CV 2 |
|---|---|---|
| Cosmetic formulation | ||
| Batch No F1 | 0.0511 (±0.0015) | 2.93 |
| Batch No F2 | 0.0507 (±0.0011) | 2.16 |
| Batch No F3 | 0.0491 (±0.0016) | 3.25 |
| Cosmetic cream | ||
| Batch No C1 | 0.00491 (±0.00018) | 3.7 |
| Batch No C2 | 0.00501 (±0.00013) | 2.6 |
| Batch No C3 | 0.00509 (±0.00021) | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raikou, V.; Kalogria, E.; Varvaresou, A.; Tsirivas, E.; Panderi, I. Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection. Separations 2021, 8, 125. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8080125
Raikou V, Kalogria E, Varvaresou A, Tsirivas E, Panderi I. Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection. Separations. 2021; 8(8):125. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8080125
Chicago/Turabian StyleRaikou, Vasiliki, Eleni Kalogria, Athanasia Varvaresou, Efthimios Tsirivas, and Irene Panderi. 2021. "Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection" Separations 8, no. 8: 125. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8080125