Efficient Separation of Heavy Metals by Magnetic Nanostructured Beads
Abstract
:1. Introduction
2. Results and Discussion
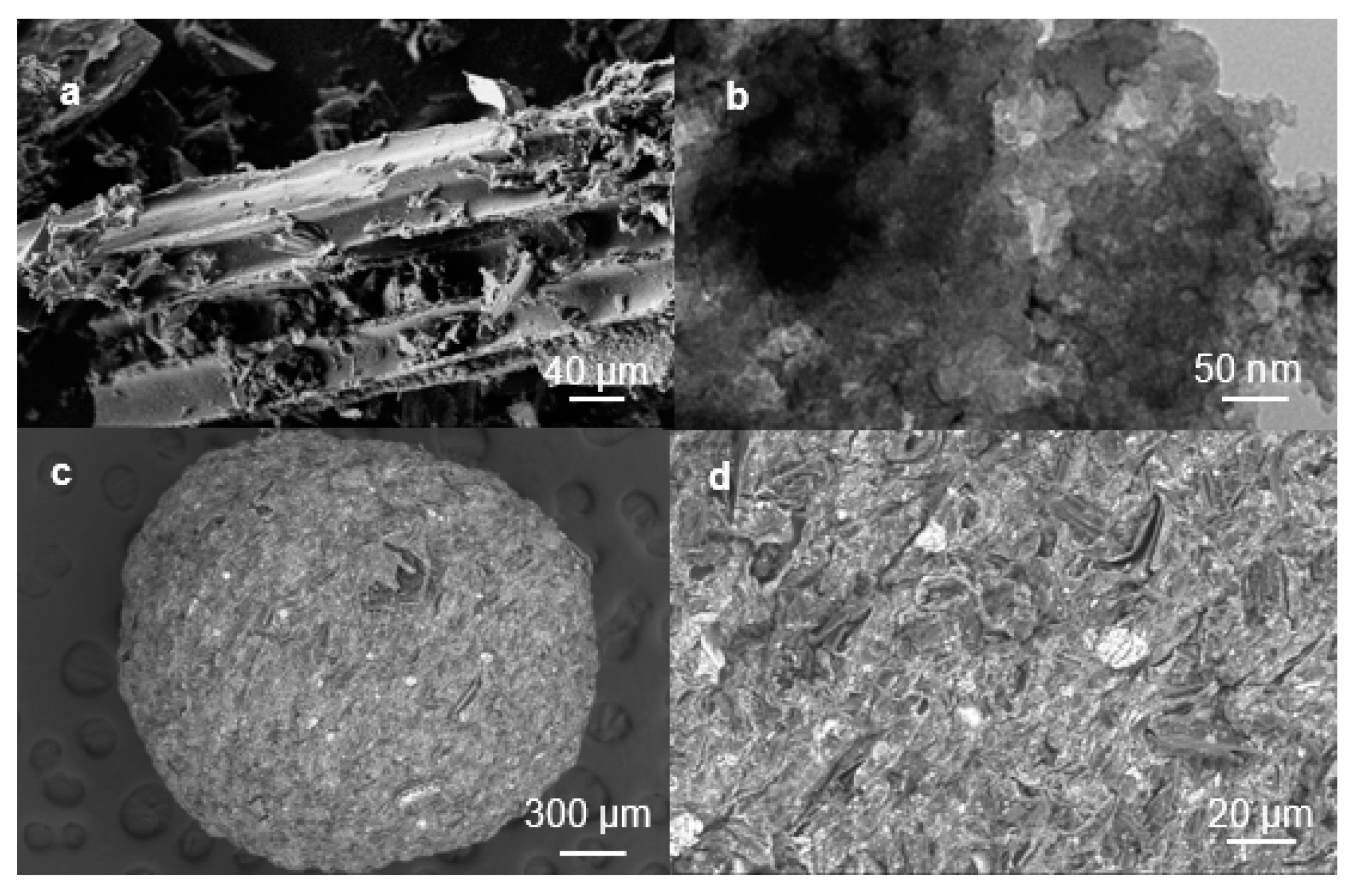
2.1. Morphology of Magnetic Beads
2.2. Structural and Textural Characterization
2.3. Specific Surface Are and Pore-Distribution
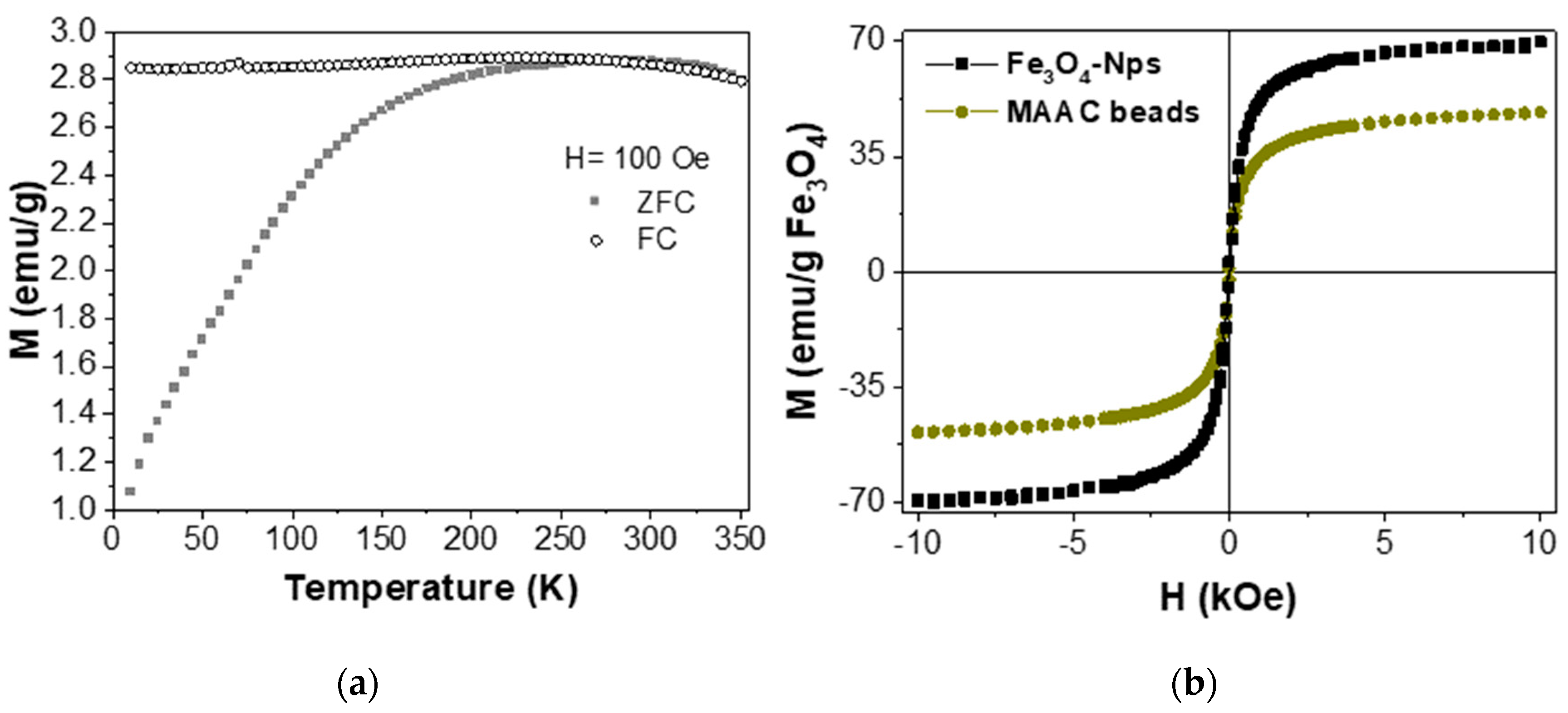
2.4. Magnetic Properties of MAAC Beads
3. Adsorption Study
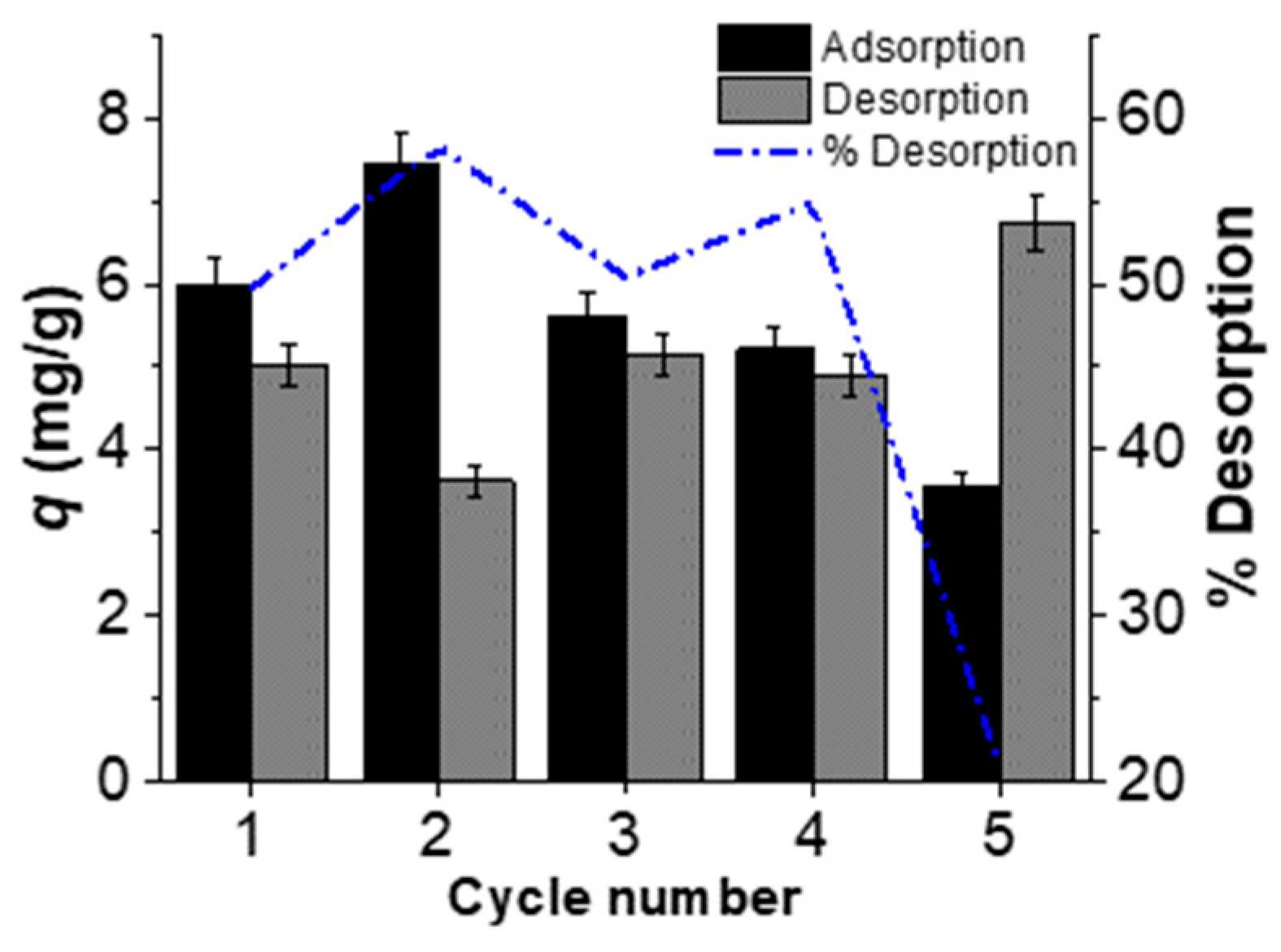
3.1. Desorption and Reusability
3.2. Effect of pH
3.3. Adsorption of Mono and Ternary Systems
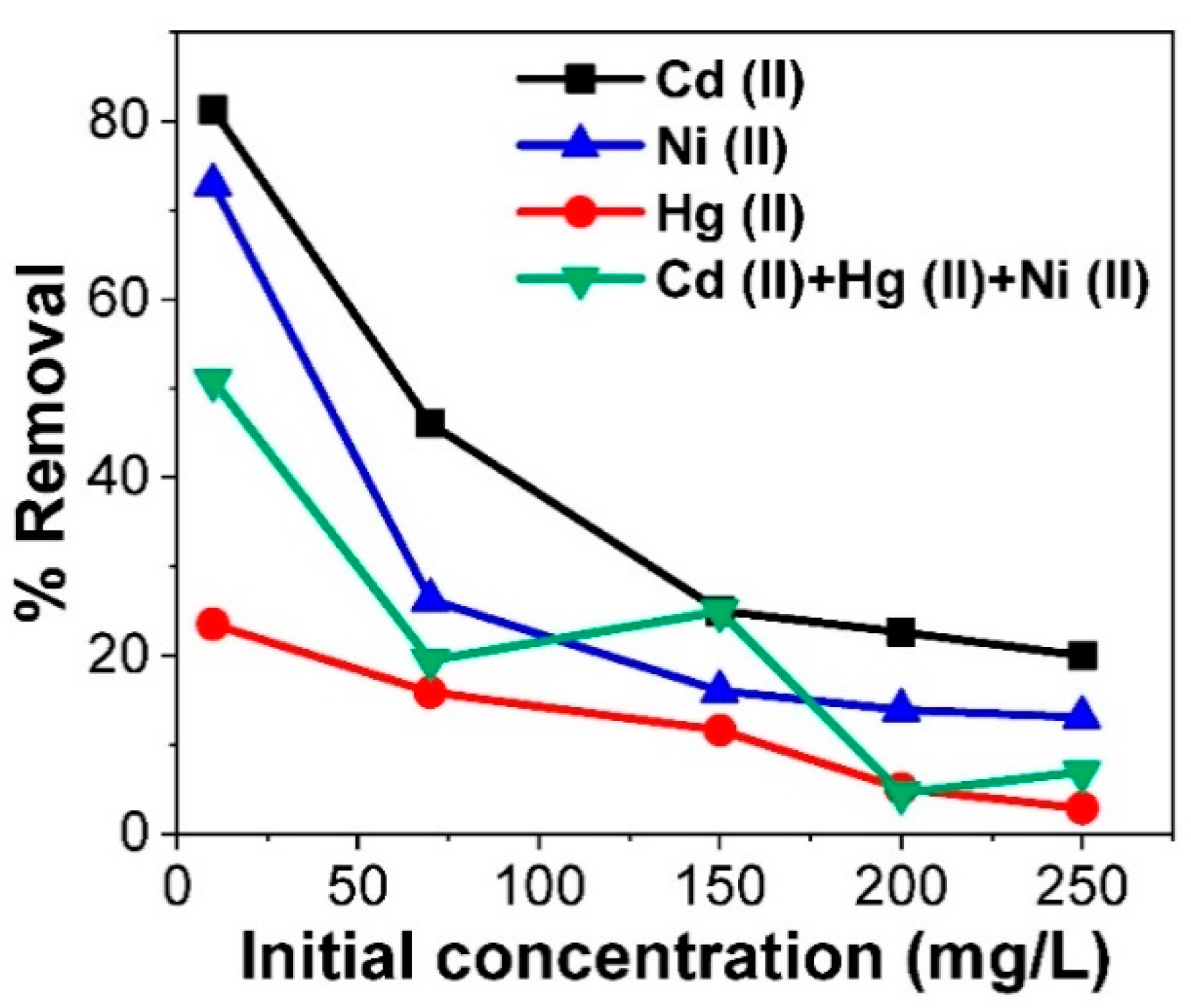
3.4. Competitive Adsorption Evaluation
3.5. Adsorption Isotherms
4. Materials and Methods
4.1. Synthesis of MAAC Beads
4.2. Effect of pH
4.3. Adsorption Study
4.4. Desorption and Reusability
5. Characterization
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.Y.; Desmarais, T.; Costa, M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 537–554. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Bryan, G.W. The effects of heavy metals (other than mercury) on marine and estuarine organisms. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1971, 177, 389–410. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.; Boaventura, R.A. Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem. 2005, 40, 3267–3275. [Google Scholar] [CrossRef]
- Lagoa, R.; Rodrigues, J.R. Evaluation of Dry Protonated Calcium Alginate Beads for Biosorption Applications and Studies of Lead Uptake. Appl. Biochem. Biotechnol. 2007, 143, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Arica, M.Y.; Bayramoǧlu, G.; Yilmaz, M.; Bektaş, S.; Genç, Ö. Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J. Hazard. Mater. 2004, 109, 191–199. [Google Scholar] [PubMed]
- Bayramoğlu, G.; Tüzün, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury(II), cadmium(II) and lead(II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Silva, R.M.P.; Manso, J.P.H.; Rodrigues, J.R.; Lagoa, R. A comparative study of alginate beads and an ion-exchange resin for the removal of heavy metals from a metal plating effluent. J. Environ. Sci. Health Part A 2008, 43, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Venault, A.; Vachoud, L.; Pochat, C.; Bouyer, D.; Faur, C. Elaboration of chitosan/activated carbon composites for the removal of organic micropollutants from waters. Environ. Technol. 2008, 29, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, D.-H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Fiol, N.; Villaescusa, I.; Bollinger, J.-C. Arsenic removal by a waste metal (hydr)oxide entrapped into calcium alginate beads. J. Hazard. Mater. 2009, 164, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, R.; Li, L.; Fang, Y.; Appelqvist, I.; Mendes, E. Drying and Rehydration of Calcium Alginate Gels. Food Biophys. 2008, 3, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpoor, F.; Shojaosadati, S.A.; Mousavi, S.Z. Magnetic silica coated iron carbide/alginate beads: Synthesis and application for adsorption of Cu(II) from aqueous solutions. Int. J. Biol. Macromol. 2019, 128, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Bée, A.; Talbot, D.; Abramson, S.; Dupuis, V. Magnetic alginate beads for Pb(II) ions removal from wastewater. J. Colloid Interface Sci. 2011, 362, 486–492. [Google Scholar] [CrossRef] [PubMed]
- de Alves, L.C.; Yáñez-Vilar, S.; Piñeiro, Y.; Rivas, J. Novel Magnetic Nanostructured Beads for Cadmium(II) Removal. Nanomaterials 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Hammouda, S.; Adhoum, N.; Monser, L. Synthesis of magnetic alginate beads based on magnetite nanoparticles for the removal of 3-methylindole from aqueous solution using Fenton process. J. Hazard. Mater. 2015, 294, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Hao, L.; Jiang, W.; Gong, X.; Hu, Y.; Chen, Z. Preparation of water-soluble magnetite nanocrystals through hydrothermal approach. J. Magn. Magn. Mater. 2007, 308, 210–213. [Google Scholar] [CrossRef]
- Khoo, K.-M.; Ting, Y.-P. Biosorption of gold by immobilized fungal biomass. Biochem. Eng. J. 2001, 8, 51–59. [Google Scholar] [CrossRef]
- Tripathi, A.; Meload, J.S.; D’Souza, S.F. Uranium (VI) recovery from aqueous medium using novel floating macroporous alginate-agarose-magnetite cryobeads. J. Hazard. Mater. 2013, 246, 87–95. [Google Scholar] [CrossRef]
- Waller, P.A.; Pickering, W.F. Chemical Speciation & Bioavailability The effect of pH on the lability of lead and cadmium sorbed on humic acid particles The effect of pH on the lability of lead and cadmium sorbed on humic acid particles. Chem. Speciat. Bioavailab. 1993, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Budimirovic, D.; Bajić, Z.; Velickovic, Z.; Milosevic, A.; Nikolic, J.B.; Drmanic, S.; Marinkovic, A. Removal of heavy metals from water using multistage functionalized multiwall carbon nanotubes. J. Serbian Chem. Soc. 2017, 82, 1175–1191. [Google Scholar] [CrossRef]
- Zouboulis, A.; Kydros, K.; Zouboulis, A.I.; Kydros, K.A. Use of red mud for toxic metals removal: The case of nickel. Artic. J. Chem. Technol. Biotechnol. 2007, 58, 95–101. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Pettignano, A.; Villaescusa, I. Mercury(II) removal from aqueous solution by sorption onto alginate, pectate and polygalacturonate calcium gel beads. A kinetic and speciation based equilibrium study. React. Funct. Polym. 2013, 73, 207–217. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, X.-Y.; Niu, C.; Chen, L.; Deng, J.-H.; Zhang, X.-R.; Niu, Q.-Y. Copper(II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem. Eng. J. 2012, 185–186, 100–107. [Google Scholar] [CrossRef]
- Jeon, C.; Park, J.Y.; Yoo, Y.J. Novel immobilization of alginic acid for heavy metal removal. Biochem. Eng. J. 2002, 11, 159–166. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Competitive Adsorption of Heavy Metal Ionsfrom Aqueous Solutions onto Activated Carbonand Agricultural Waste Materials. Pol. J. Environ. Stud. 2019, 29, 749–761. [Google Scholar] [CrossRef]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Villaescusa, I. Simultaneous adsorption behavior of heavy metals onto microporous olive stones activated carbon: Analysis of metal interactions. Euro-Mediterr. J. Environ. Integr. 2017, 2, 19. [Google Scholar] [CrossRef]



| Samples | BET Surface Area (SBET) 1 (m2/g) | Pore Volume (cm3/g) | Pore Diameter 2 (nm) |
|---|---|---|---|
| Commercial AC | 849.32 | 0.28 | 1.26 |
| MAAC | 107.13 | 0.075 | 1.24 |
| Initial Concentration (mg/L) | 10 | 70 | 150 | 200 | 250 | |
|---|---|---|---|---|---|---|
| Adsorbent | Metal Ions | Evaluation Ratios | ||||
| MAAC | Cd(II) | 1.08 | 0.58 | 1.10 | 0.35 | 0.56 |
| Hg(II) | 0.14 | 0.66 | 1.46 | 0.44 | 1.36 | |
| Ni(II) | 1.05 | 0.63 | 1.38 | 0.20 | 0.26 | |
| Cd(II) + Hg(II) + Ni(II) | 0.68 | 0.71 | 1.48 | 0.32 | 0.48 | |
| Adsorption System | Metal Ions | Langmuir Parameters | Freundlich Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | RL | R2 | n | 1/n | KF (mg1−(1/n)L1/ng−1) | R2 | ||
| Cd(II) | Cd(II) | 59.17 | 0.048 | 0.103 | 0.981 | 2.52 | 0.397 | 7.09 | 0.983 |
| Hg(II) | Hg(II) | 25.00 | 0.040 | 0.118 | 0.922 | 3.94 | 0.254 | 5.08 | 0.865 |
| Ni(II) | Ni(II) | 37.04 | 0.031 | 0.144 | 0.945 | 2.79 | 0.358 | 4.82 | 0.995 |
| Cd(II) + Hg(II) + Ni(II) | Cd(II) | 56.18 | 0.029 | 0.339 | 0.758 | 2.49 | 0.401 | 6.11 | 0.889 |
| Hg(II) | 181.82 | 0.007 | 0.164 | 0.681 | 1.50 | 0.668 | 3.35 | 0.901 | |
| Ni(II) | 172.71 | 0.001 | 0.456 | 0.561 | 1.14 | 0.878 | 0.35 | 0.953 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Castro Alves, L.; Yáñez-Vilar, S.; Piñeiro-Redondo, Y.; Rivas, J. Efficient Separation of Heavy Metals by Magnetic Nanostructured Beads. Inorganics 2020, 8, 40. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060040
de Castro Alves L, Yáñez-Vilar S, Piñeiro-Redondo Y, Rivas J. Efficient Separation of Heavy Metals by Magnetic Nanostructured Beads. Inorganics. 2020; 8(6):40. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060040
Chicago/Turabian Stylede Castro Alves, Lisandra, Susana Yáñez-Vilar, Yolanda Piñeiro-Redondo, and José Rivas. 2020. "Efficient Separation of Heavy Metals by Magnetic Nanostructured Beads" Inorganics 8, no. 6: 40. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics8060040





