Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Animal Sample
2.3. Study Design
2.4. Sample Calculation
2.5. Randomization and Allocation Concealment
2.6. Implants Characteristics and Bioactivation of the Implant Surface with Argon Plasma
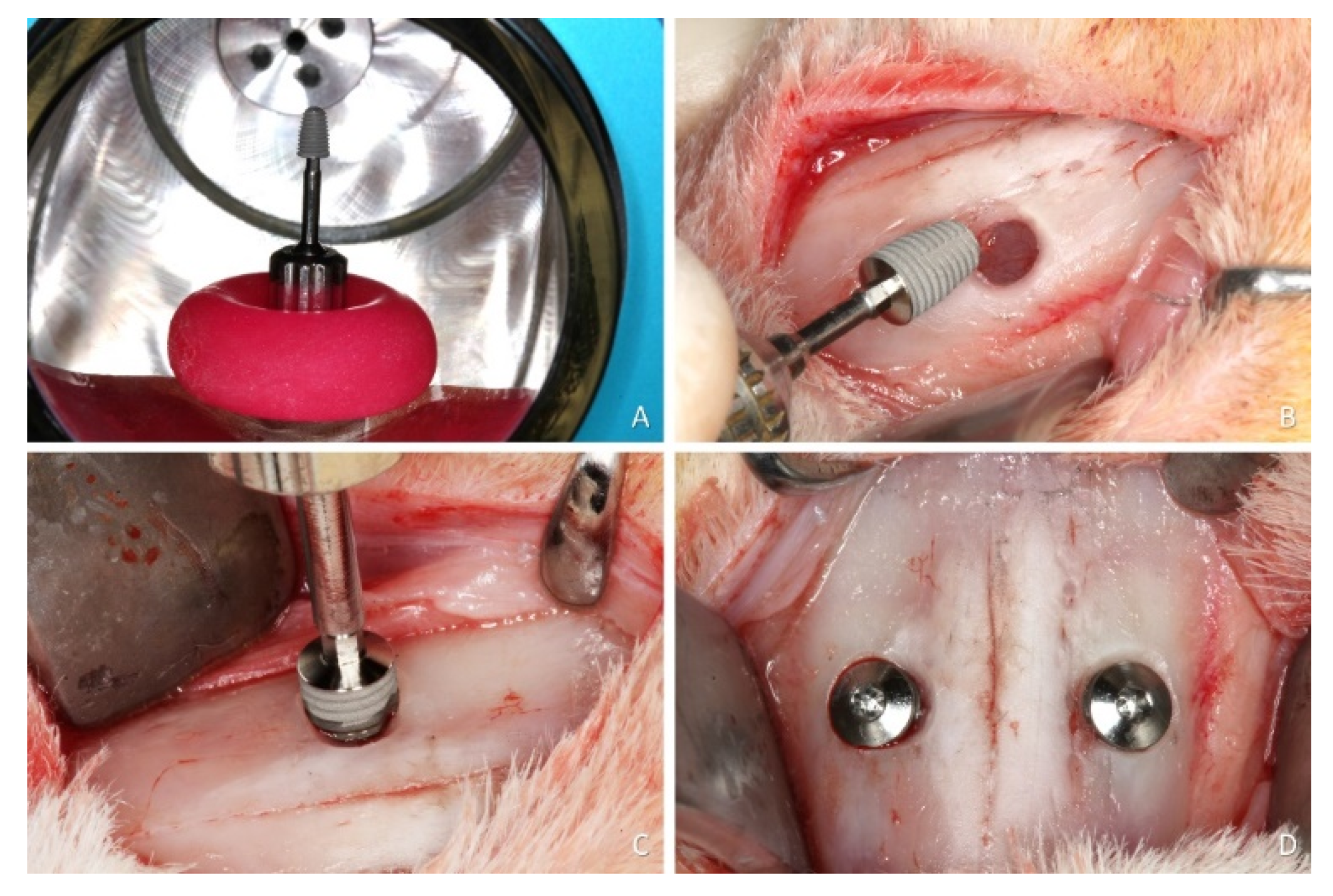
2.7. Surgical Procedures
2.8. Maintenance Care
2.9. Euthanasia
2.10. Histological Preparation
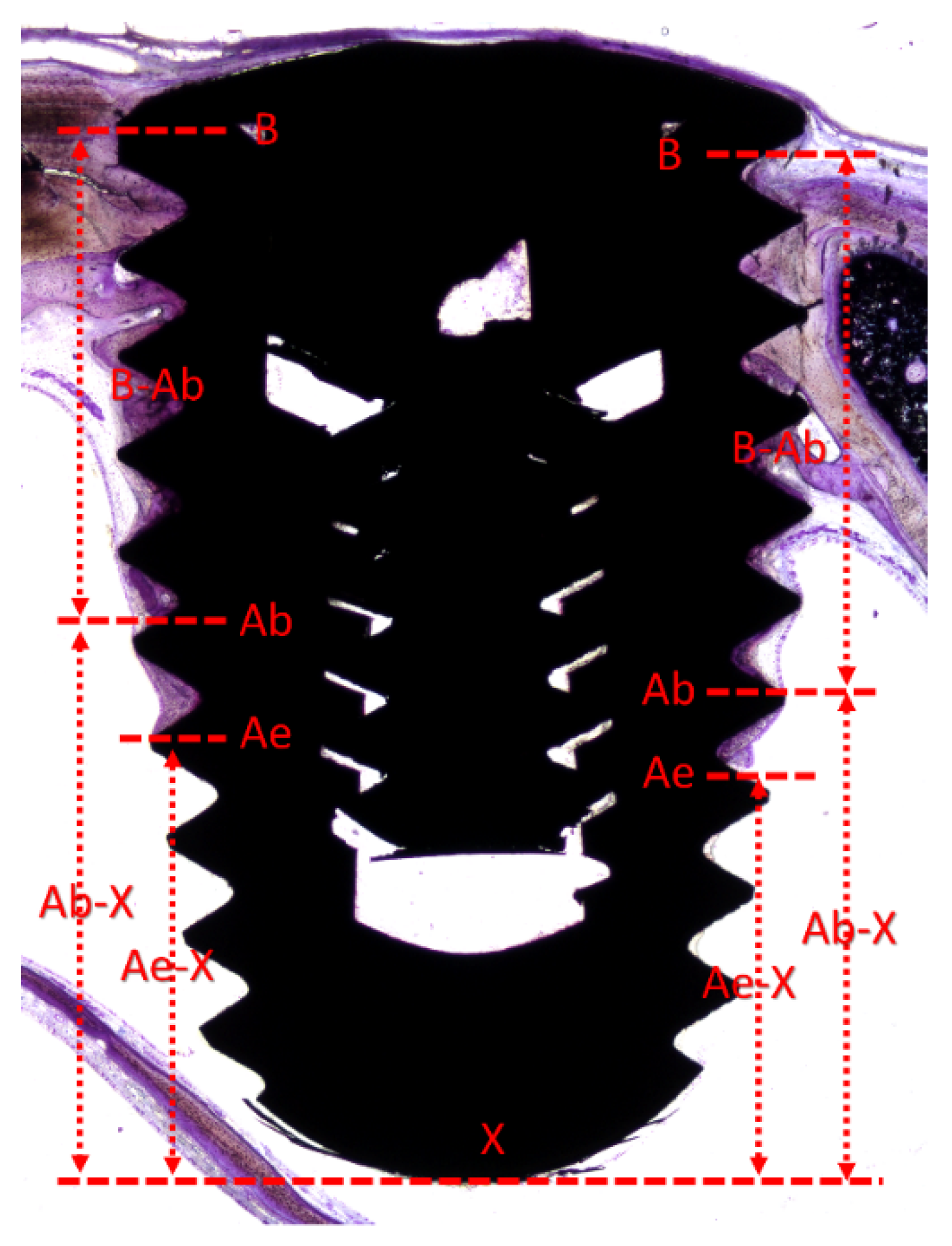
2.11. Histometric Evaluations
2.12. Data Analysis
3. Results
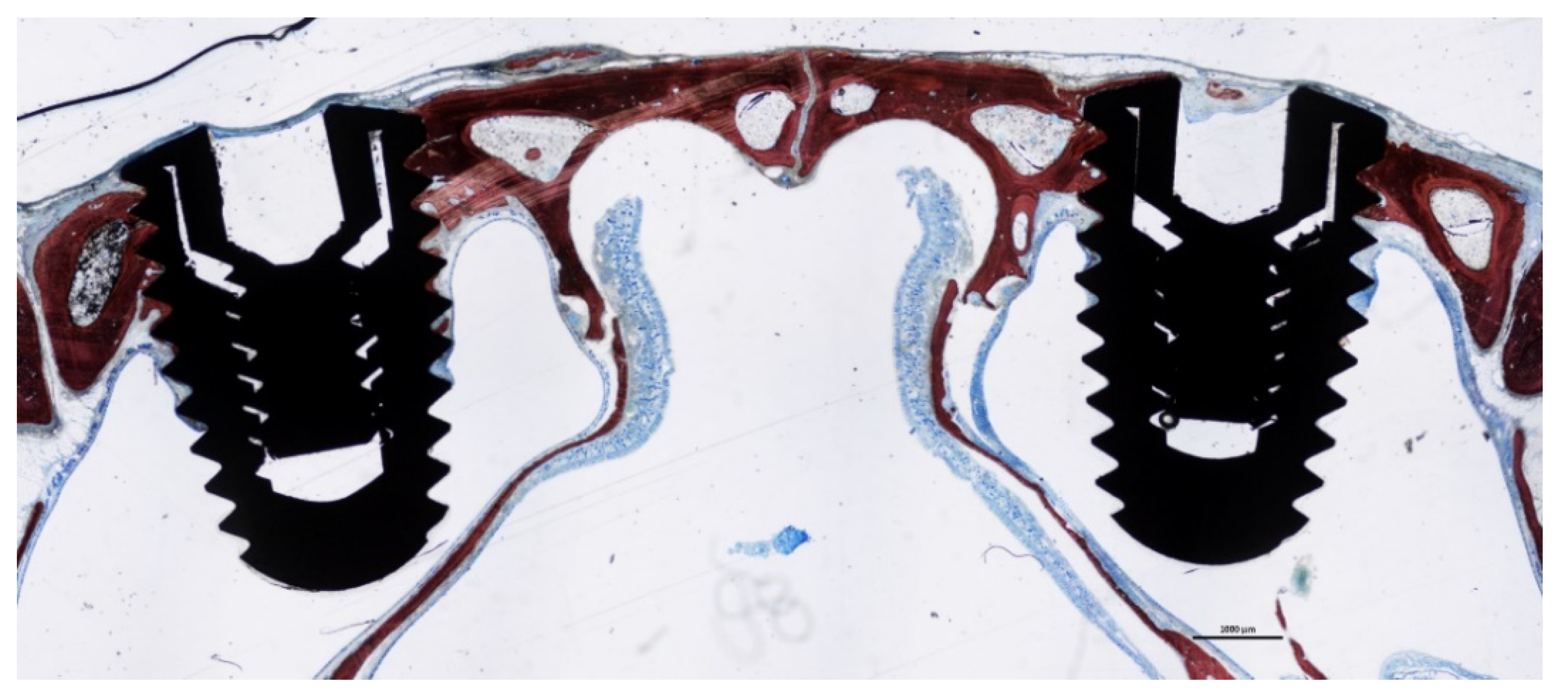
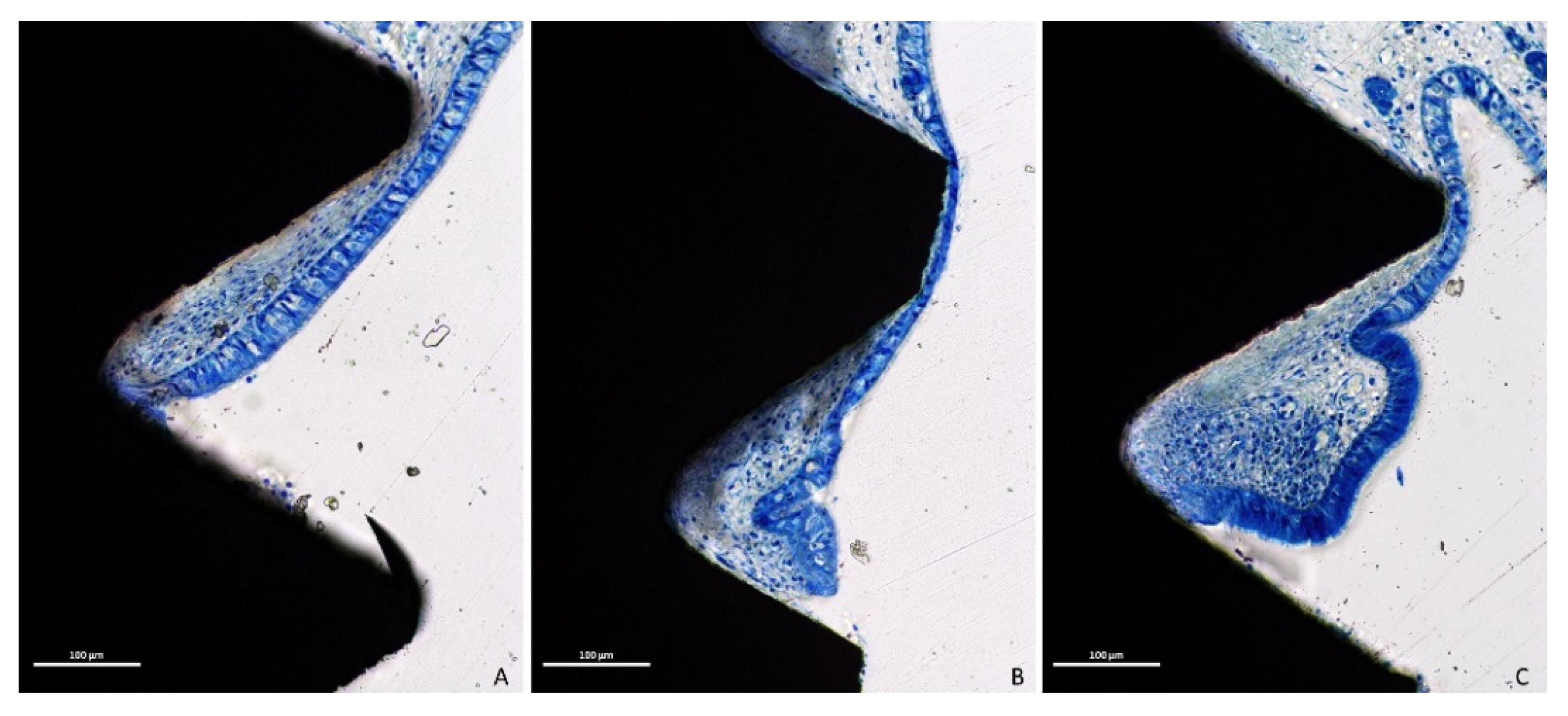
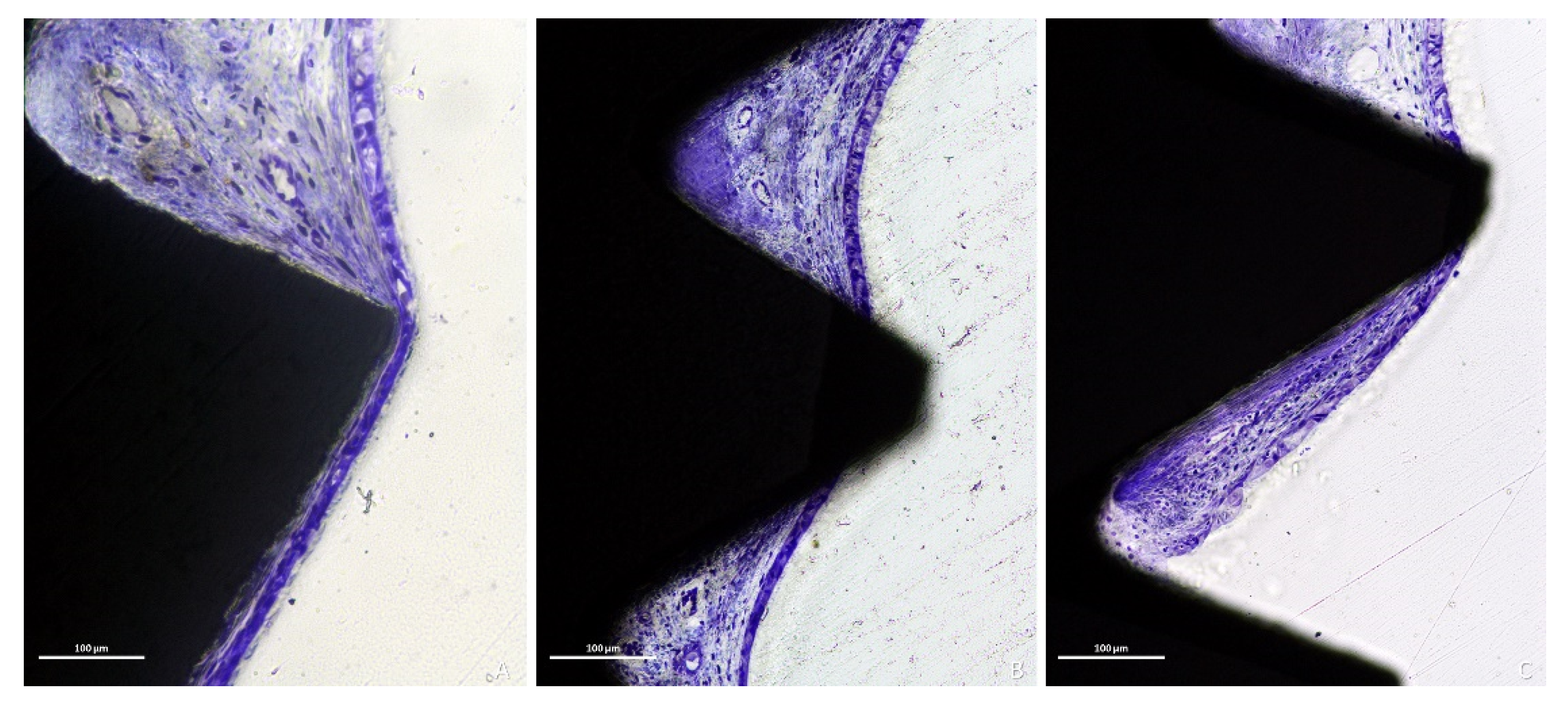
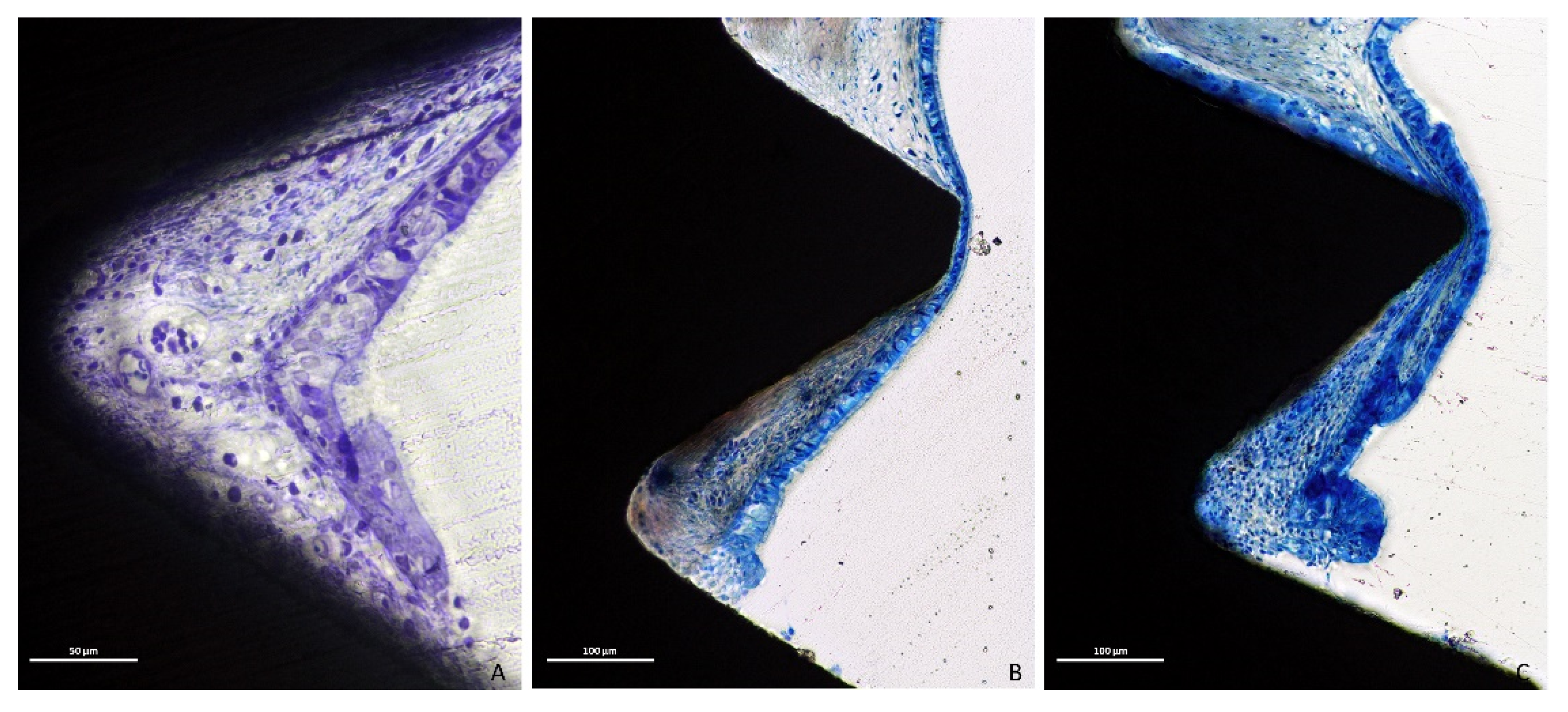
3.1. Descriptive Histological Evaluation
3.2. Histometric Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 216–240. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Wallace, S.S.; Testori, T. Long-term implant survival in the grafted maxillary sinus: A systematic review. Int. J. Periodontics Restor. Dent. 2013, 33, 773–783. [Google Scholar] [CrossRef]
- Lozano-Carrascal, N.; Salomó-Coll, O.; Gehrke, S.A.; Calvo-Guirado, J.L.; Hernández-Alfaro, F.; Gargallo-Albiol, J. Radiological evaluation of maxillary Sinus anatomy: A cross-sectional study of 300 patients. Ann. Anat. 2017, 214, 1–8. [Google Scholar] [CrossRef]
- Kawakami, S.; Botticelli, D.; Nakajima, Y.; Sakuma, S.; Baba, S. Anatomical analyses for maxillary sinus floor augmentation with a lateral approach: A cone beam computed tomography study. Ann. Anat. 2019, 226, 29–34. [Google Scholar] [CrossRef]
- Sakuma, S.; Ferri, M.; Imai, H.; Fortich Mesa, N.; Blanco Victorio, D.J.; Apaza Alccayhuaman, K.A.; Botticelli, D. Involvement of the maxillary sinus ostium (MSO) in the edematous processes after sinus floor augmentation: A cone-beam computed tomographic study. Int. J. Implant Dent. 2020, 6, 35. [Google Scholar] [CrossRef]
- Omori, Y.; Nakajima, Y.; Imai, H.; Yonezawa, D.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of Anatomical Parameters on the Dimensions of the Subantral Space and Sinus Mucosa Thickening after Sinus Floor Elevation. A Retrospective Cone Beam Computed Tomography Study. Dent. J. 2021, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Ferrín, L.; Galán-Gil, S.; Rubio-Serrano, M.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Maxillary sinus septa: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e383–e386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Omori, Y.; Kanayama, M.; Hirota, A.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Sinus Mucosa Thickness Changes and Ostium Involvement After Maxillary Sinus Floor Elevation in Sinus with Septa. A Cone Beam Computed Tomography Study. Dent. J. 2021, 9, 82. [Google Scholar] [CrossRef]
- Kuligowski, P.; Jaroń, A.; Preuss, O.; Gabrysz-Trybek, E.; Bladowska, J.; Trybek, G. Association between Odontogenic and Maxillary Sinus Conditions: A Retrospective Cone-Beam Computed Tomographic Study. J. Clin. Med. 2021, 10, 2849. [Google Scholar] [CrossRef]
- Malec, M.; Smektala, T.; Tutak, M.; Trybek, G. Sporniak-Tutak, K. Maxillary Sinus Septa Prevalence and Morphology: Computed Tomography Based Analysis. Int. J. Morphol. 2015, 33, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Grafting of deproteinized bone particles inhibits bone resorption after maxillary sinus floor elevation. Clin. Oral Implant. Res. 2004, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Cricchio, G.; Palma, V.C.; Faria, P.E.; de Oliveira, J.A.; Lundgren, S.; Sennerby, L.; Salata, L.A. Histological findings following the use of a space-making device for bone reformation and implant integration in the maxillary sinus of primates. Clin. Implant Dent. Relat. Res. 2009, 11 (Suppl. 1), e14–e22. [Google Scholar] [CrossRef]
- Cricchio, G.; Palma, V.C.; Faria, P.E.; de Olivera, J.A.; Lundgren, S.; Sennerby, L.; Salata, L.A. Histological outcomes on the development of new space-making devices for maxillary sinus floor augmentation. Clin. Implant Dent. Relat. Res. 2011, 13, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, M.; Botticelli, D.; de Oliveira, J.A.; Scala, A.; Salata, L.A.; Lang, N.P. Use of a titanium device in lateral sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.Å.; Isaksson, S.; Adolfsson, E.; Lindh, C.; Sennerby, L. Bone regeneration using a hollow hydroxyapatite space-maintaining device for maxillary sinus floor augmentation—A clinical pilot study. Clin. Implant Dent. Relat. Res. 2012, 14, 575–584. [Google Scholar] [CrossRef]
- Ellegaard, B.; Kølsen-Petersen, J.; Baelum, V. Implant therapy involving maxillary sinus lift in periodontally compromised patients. Clin. Oral Implant. Res. 1997, 8, 305–315. [Google Scholar] [CrossRef]
- Lundgren, S.; Andersson, S.; Gualini, F.; Sennerby, L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin. Implant Dent. Relat. Res. 2004, 6, 165–173. [Google Scholar] [CrossRef]
- Ellegaard, B.; Baelum, V.; Kølsen-Petersen, J. Non-grafted sinus implants in periodontally compromised patients: A time-to-event analysis. Clin. Oral Implant. Res. 2006, 17, 156–164. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Faeda, R.S.; Garcia Rangel, I., Jr.; Américo de Oliveira, J.; Lang, N.P. Lack of influence of the Schneiderian membrane in forming new bone apical to implants simultaneously installed with sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Botticelli, D.; Rangel, I.G., Jr.; de Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant. Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.D.; de Lima, V.N.; Faverani, L.P.; de Mendonça, M.R.; Okamoto, R.; Pellizzer, E.P. Maxillary sinus lift surgery-with or without graft material? A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 1570–1576. [Google Scholar] [CrossRef] [Green Version]
- Cricchio, G.; Sennerby, L.; Lundgren, S. Sinus bone formation and implant survival after sinus membrane elevation and implant placement: A 1- to 6-year follow-up study. Clin. Oral Implant. Res. 2011, 22, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Silva, E.R.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P. Histologic and Micro-CT Analyses at Implants Placed Immediately After Maxillary Sinus Elevation Using Large or Small Xenograft Granules: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implants 2020, 35, 739–748. [Google Scholar] [CrossRef]
- Schwarz, L.; Schiebel, V.; Hof, M.; Ulm, C.; Watzek, G.; Pommer, B. Risk Factors of Membrane Perforation and Postoperative Complications in Sinus Floor Elevation Surgery: Review of 407 Augmentation Procedures. J. Oral Maxillofac. Surg. 2015, 73, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Taschieri, S.; Wallace, S.S. Risk factors in lateral window sinus elevation surgery. Periodontology 2000 2019, 81, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Yu, S.H.; Tavelli, L.; Wang, H.L. Perforation Risk Assessment in Maxillary Sinus Augmentation with Lateral Wall Technique. Int. J. Periodontics Restor. Dent. 2020, 40, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lindstrom, J.; Rockler, B. An experimental and clinical study of osseointegrated implants penetrating the nasal cavity and maxillary sinus. J. Oral Maxillofac. Surg. 1984, 42, 497–505. [Google Scholar] [CrossRef]
- Nooh, N. Effect of Schneiderian membrane perforation on posterior maxillary implant survival. J. Int. Oral Health 2013, 5, 28–34. [Google Scholar]
- Kato, S.; Botticelli, D.; De Santis, E.; Kanayama, M.; Ferreira, S.; Rangel-Garcia, I., Jr. Sinus mucosa thinning and perforation after sinus augmentation. A histological study in rabbits. Oral Maxillofac. Surg. 2021, 4. epub ahead of print. [Google Scholar] [CrossRef]
- Miki, M.; Botticelli, D.; Silva, E.R.; Xavier, S.P.; Baba, S. Incidence of sinus mucosa perforations during healing after sinus lifting using deproteinized bovine bone mineral as grafting material. A histological evaluation in a rabbit model. Int. J. Oral Maxillofac. Implants 2021, 36, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin. Oral Implant. Res. 2004, 15, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Favero, R.; Lang, N.P.; Salata, L.A.; Martins Neto, E.C.; Caroprese, M.; Botticelli, D. Sequential healing events of osseointegration at UnicCa(®) and SLActive(®) implant surfaces: An experimental study in the dog. Clin. Oral Implant. Res. 2016, 27, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bengazi, F.; Lang, N.P.; Canciani, E.; Viganò, P.; Velez, J.U.; Botticelli, D. Osseointegration of implants with dendrimers surface characteristics installed conventionally or with Piezosurgery®. A comparative study in the dog. Clin. Oral Implant. Res. 2014, 25, 10–15. [Google Scholar] [CrossRef]
- Caneva, M.; Lang, N.P.; Calvo Guirado, J.L.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations. An experimental study in rabbits. Clin. Oral Implant. Res. 2015, 26, 293–299. [Google Scholar] [CrossRef]
- Akimoto, K.; Becker, W.; Donath, K.; Becker, B.E.; Sanchez, R. Formation of bone around titanium implants placed into zero wall defects: Pilot project using reinforced e-PTFE membrane and autogenous bone grafts. Clin. Implant Dent. Relat. Res. 1999, 1, 98–104. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Persson, L.G.; Lindhe, J. Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 448–455. [Google Scholar] [CrossRef]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implant. Res. 2014, 25, 124–131. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Cassinelli, C.; Götz, W.; Tarnow, D. Plasma of argon accelerates murine fibroblast adhesion in early stages of titanium disk colonization. Int. J. Oral Maxillofac. Implant. 2013, 28, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [Green Version]
- Ismail, F.S.; Rohanizadeh, R.; Atwa, S.; Mason, R.S.; Ruys, A.J.; Martin, P.J.; Bendavid, A. The influence of surface chemistry and topography on the contact guidance of MG63 osteoblast cells. J. Mater. Sci. Mater. Med. 2007, 18, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Zhuang, L.F.; Liu, X.; Wieland, M.; Zhang, Z.Y.; Zhang, Z.Y. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J. Biomed. Mater. Res. A 2010, 93, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kabaso, D.; Gongadze, E.; Perutková, S.; Matschegewski, C.; Kralj-Iglic, V.; Beck, U.; van Rienen, U.; Iglic, A. Mechanics and electrostatics of the interactions between osteoblasts and titanium surface. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Camacho, F.; Peñarrocha, D.; Tallarico, M.; Perez, S.; Canullo, L. Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: A randomized controlled histologic study. Clin. Oral Implant. Res. 2017, 28, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Rakic, M.; Sculean, A.; Miron, R.; Muzzi, M.; Carossa, S.; Mussano, F. Effects of argon plasma treatment on the osteoconductivity of bone grafting materials. Clin. Oral Investig. 2020, 24, 2611–2623. [Google Scholar] [CrossRef]
- Canullo, L.; Tallarico, M.; Botticelli, D.; Alccayhuaman, K.A.A.; Martins Neto, E.C.; Xavier, S.P. Hard and soft tissue changes around implants activated using plasma of argon: A histomorphometric study in dog. Clin. Oral Implant. Res. 2018, 29, 389–395. [Google Scholar] [CrossRef]
- Hirota, A.; Yamada, Y.; Canullo, L.; Xavier, S.P.; Baba, S. Bioactivation of Bovine Bone Matrix Using Argon Plasma: An Experimental Study for Sinus Augmentation in Rabbits. Int. J. Oral Maxillofac. Implant. 2020, 35, 731–738. [Google Scholar] [CrossRef]
- Tanaka, K.; Botticelli, D.; Canullo, L.; Baba, S.; Xavier, S.P. New bone ingrowth into β-TCP/HA graft activated with argon plasma: A histomorphometric study on sinus lifting in rabbits. Int. J. Implant Dent. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Yonezawa, D.; Silva, E.R.; Xavier, S.P. The impact on the healing of bioactivation with argon plasma of a xenogeneic graft with adequate fixation but poor adaptation to the recipient site: An experimental study in rabbits. Int. J. Oral Maxillofac. Implant. 2021, 36, 703–714. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Ferreira, S.; Rangel Garcia, I., Jr.; Caneva, M.; Botticelli, D. Healing at implants installed concurrently to maxillary sinus floor elevation with Bio-Oss(®) or autologous bone grafts. A histo-morphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 503–511. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Pesce, P.; Nakajima, Y.; Yonezawa, D.; Mussano, F. Surface bio-functionalization using plasma of argon could alter microbiological and topographic surface analysis of dental implants? Ann. Anat. 2020, 230, 151489. [Google Scholar] [CrossRef]
- Iida, T.; Carneiro Martins Neto, E.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Lang, N.P.; Xavier, S.P. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin Oral Implants Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef]
- Ting, M.; Rice, J.G.; Braid, S.M.; Lee, C.Y.S.; Suzuki, J.B. Maxillary Sinus Augmentation for Dental Implant Rehabilitation of the Edentulous Ridge: A Comprehensive Overview of Systematic Reviews. Implant Dent. 2017, 26, 438–464. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Volume changes of maxillary sinus augmentations over time: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 881–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coopman, R.; Fennis, J.; Ghaeminia, H.; Van de Vyvere, G.; Politis, C.; Hoppenreijs, T.J.M. Volumetric osseous changes in the completely edentulous maxilla after sinus grafting and lateral bone augmentation: A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Lang, N.P.; Iida, T.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the position of the antrostomy in sinus floor elevation assessed with cone-beam computed tomography: A randomized clinical trial. J. Investig. Clin. Dent. 2018, 9, e12362. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Lang, N.P.; Ferri, M.; Alccayhuaman, K.A.A.; Botticelli, D. Influence of the height of the antrostomy in sinus floor elevation assessed by cone beam computed tomography- a randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2019, 34, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Lang, N.P.; Ferri, M.; Mesa, N.F.; Alccayhuaman, K.A.A.; Botticelli, D. Tomographic evaluation of the influence of the placement of a collagen membrane subjacent to the sinus mucosa during maxillary sinus floor augmentation: A randomized clinical trial. Int. J. Implant Dent. 2019, 5, 31. [Google Scholar] [CrossRef]
- Imai, H.; Lang, N.P.; Ferri, M.; Hirota, A.; Apaza Alccayhuaman, A.A.; Botticelli, D. Tomographic assessment on the influence on dimensional variations of the use of a collagen membrane to protect the antrostomy after maxillary sinus floor augmentation. A randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2020, 35, 350–356. [Google Scholar] [CrossRef]
- Caneva, M.; Lang, N.P.; Garcia Rangel, I.J.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss(®) or Gingistat(®) collagen sponge: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Silva, E.R.; Lang, N.P.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P. Histological and micro-computed tomography evaluations of newly formed bone after maxillary sinus augmentation using a xenograft with similar density and mineral content of bone: An experimental study in rabbits. Clin. Exp. Dent. Res. 2018, 4, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragucci, G.M.; Elnayef, B.; Suárez-López Del Amo, F.; Wang, H.L.; Hernández-Alfaro, F.; Gargallo-Albiol, J. Influence of exposing dental implants into the sinus cavity on survival and complications rate: A systematic review. Int. J. Implant Dent. 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Choi, B.H.; Zhu, S.J.; Lee, S.H.; Huh, J.Y.; You, T.M.; Lee, H.J.; Li, J. The effects of exposing dental implants to the maxillary sinus cavity on sinus complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 602–605. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, B.; Liang, X.; Ma, G. Experimental study on penetration of dental implants into the maxillary sinus in different depths. J. Appl. Oral Sci. 2013, 21, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Elhamruni, L.M.; Marzook, H.A.; Ahmed, W.M.; Abdul-Rahman, M. Experimental study on penetration of dental implants into the maxillary sinus at different depths. Oral Maxillofac. Surg. 2016, 20, 281–287. [Google Scholar] [CrossRef]
- Aimetti, M.; Massei, G.; Morra, M.; Cardesi, E.; Romano, F. Correlation between gingival phenotype and Schneiderian membrane thickness. Int. J. Oral Maxillofac. Implant. 2008, 23, 1128–1132. [Google Scholar]




| B-Ab | Ab-X | Ae-X | ||
|---|---|---|---|---|
| Plasma | Mean ± SD Median (25%; 75%) | 1.97 ± 0.91 1.84 (1.22; 2.61) | 2.65 ± 0.94 2.72 (1.98; 3.39) | 1.32 ± 1.31 0.84 (0.08; 2.39) |
| Control | Mean ± SD Median (25%; 75%) | 1.81 ± 0.82 1.67 (1.17; 2.23) | 2.90 ± 0.92 3.12 (2.38; 3.64) | 1.31 ± 1.36 0.86 (0.09; 2.32) |
| Wilcoxon test | 0.678 | 0.520 | 0.891 |
| Perforations at Apex | Perforations at Threads | Thinning Sites | |
|---|---|---|---|
| Plasma | 12 | 15 | 55 |
| Control | 12 | 18 | 58 |
| Wilcoxon test | 1.000 | 1.000 | 0.826 |
| Thinning Mucosa Width | Thinnest Mucosa Width | Pristine Mucosa | ||
|---|---|---|---|---|
| Plasma | Mean ± SD Median (25%; 75%) | 14 ± 9 15 (9; 21) | 10 ± 9 7 (2; 19) | 82 ± 15 83 (68; 93) |
| Control | Mean ± SD Median (25%; 75%) | 15 ± 8 16 (9; 21) | 8 ± 7 7 (3; 11) | 79 ± 15 77 (74; 87) |
| Wilcoxon test | 0.592 | 0.608 | 0.440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, Y.; Botticelli, D.; Ferri, M.; Delgado-Ruiz, R.; Ferreira Balan, V.; Porfirio Xavier, S. Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits. Dent. J. 2021, 9, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090105
Omori Y, Botticelli D, Ferri M, Delgado-Ruiz R, Ferreira Balan V, Porfirio Xavier S. Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits. Dentistry Journal. 2021; 9(9):105. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090105
Chicago/Turabian StyleOmori, Yuki, Daniele Botticelli, Mauro Ferri, Rafael Delgado-Ruiz, Vitor Ferreira Balan, and Samuel Porfirio Xavier. 2021. "Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits" Dentistry Journal 9, no. 9: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/dj9090105







